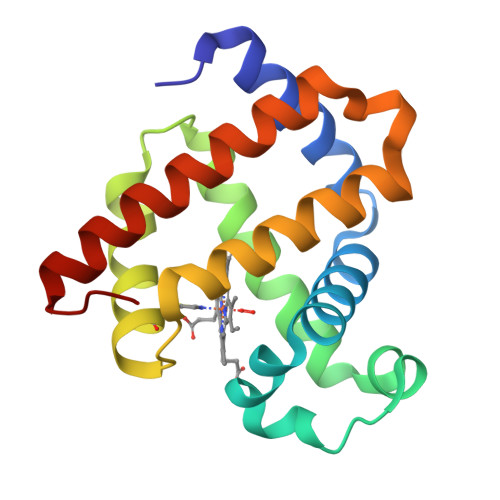
Noncanonical Heme Ligands Steer Carbene Transfer Reactivity in an Artificial Metalloenzyme*.
Pott, M., Tinzl, M., Hayashi, T., Ota, Y., Dunkelmann, D., Mittl, P.R.E., Hilvert, D.(2021) Angew Chem Int Ed Engl 60: 15063-15068
- PubMed: 33880851
- DOI: https://doi.org/10.1002/anie.202103437
- Primary Citation of Related Structures:
6Z4R, 6Z4T - PubMed Abstract:
Changing the primary metal coordination sphere is a powerful strategy for tuning metalloprotein properties. Here we used amber stop codon suppression with engineered pyrrolysyl-tRNA synthetases, including two newly evolved enzymes, to replace the proximal histidine in myoglobin with N δ -methylhistidine, 5-thiazoylalanine, 4-thiazoylalanine and 3-(3-thienyl)alanine. In addition to tuning the heme redox potential over a >200 mV range, these noncanonical ligands modulate the protein's carbene transfer activity with ethyl diazoacetate. Variants with increased reduction potential proved superior for cyclopropanation and N-H insertion, whereas variants with reduced E o values gave higher S-H insertion activity. Given the functional importance of histidine in many enzymes, these genetically encoded analogues could be valuable tools for probing mechanism and enabling new chemistries.
Organizational Affiliation:
Laboratory of Organic Chemistry, ETH Zürich, 8093, Zürich, Switzerland.