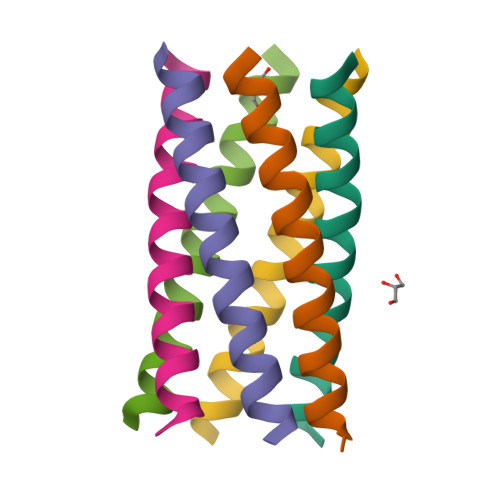
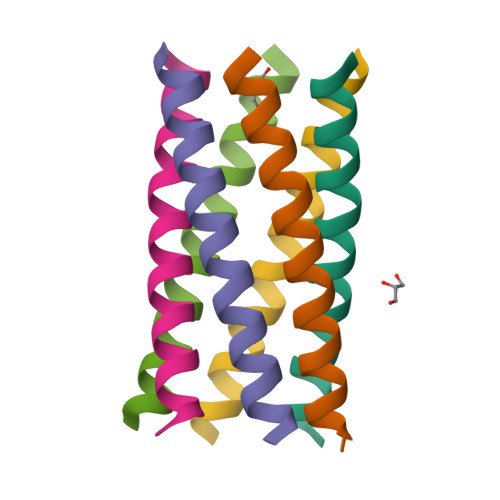
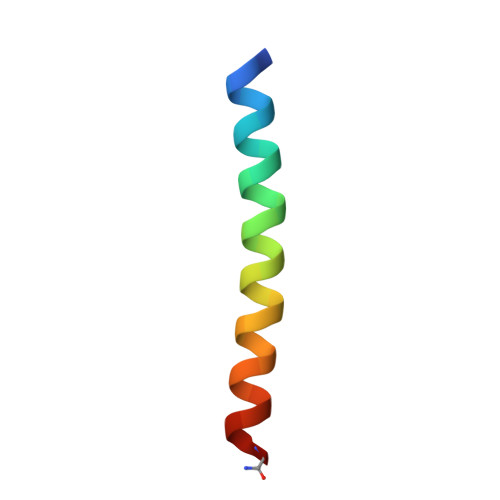
Constructing ion channels from water-soluble alpha-helical barrels.
Scott, A.J., Niitsu, A., Kratochvil, H.T., Lang, E.J.M., Sengel, J.T., Dawson, W.M., Mahendran, K.R., Mravic, M., Thomson, A.R., Brady, R.L., Liu, L., Mulholland, A.J., Bayley, H., DeGrado, W.F., Wallace, M.I., Woolfson, D.N.(2021) Nat Chem 13: 643-650
- PubMed: 33972753
- DOI: https://doi.org/10.1038/s41557-021-00688-0
- Primary Citation of Related Structures:
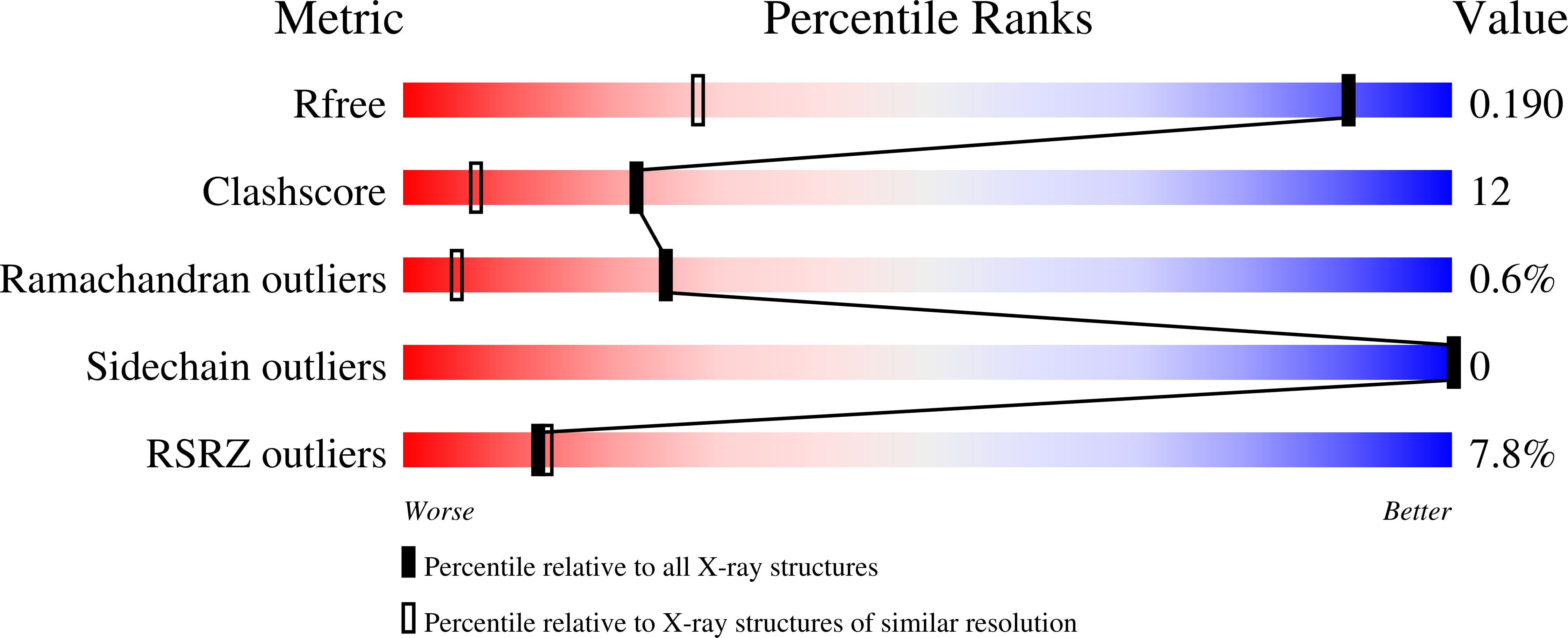
6YAZ, 6YB0, 6YB1, 6YB2 - PubMed Abstract:
The design of peptides that assemble in membranes to form functional ion channels is challenging. Specifically, hydrophobic interactions must be designed between the peptides and at the peptide-lipid interfaces simultaneously. Here, we take a multi-step approach towards this problem. First, we use rational de novo design to generate water-soluble α-helical barrels with polar interiors, and confirm their structures using high-resolution X-ray crystallography. These α-helical barrels have water-filled lumens like those of transmembrane channels. Next, we modify the sequences to facilitate their insertion into lipid bilayers. Single-channel electrical recordings and fluorescent imaging of the peptides in membranes show monodisperse, cation-selective channels of unitary conductance. Surprisingly, however, an X-ray structure solved from the lipidic cubic phase for one peptide reveals an alternative state with tightly packed helices and a constricted channel. To reconcile these observations, we perform computational analyses to compare the properties of possible different states of the peptide.
Organizational Affiliation:
School of Chemistry, University of Bristol, Bristol, UK.