Benzylaminoethyureido-Tailed Benzenesulfonamides: Design, Synthesis, Kinetic and X-ray Investigations on Human Carbonic Anhydrases.
Ali, M., Bozdag, M., Farooq, U., Angeli, A., Carta, F., Berto, P., Zanotti, G., Supuran, C.T.(2020) Int J Mol Sci 21
- PubMed: 32272689
- DOI: https://doi.org/10.3390/ijms21072560
- Primary Citation of Related Structures:
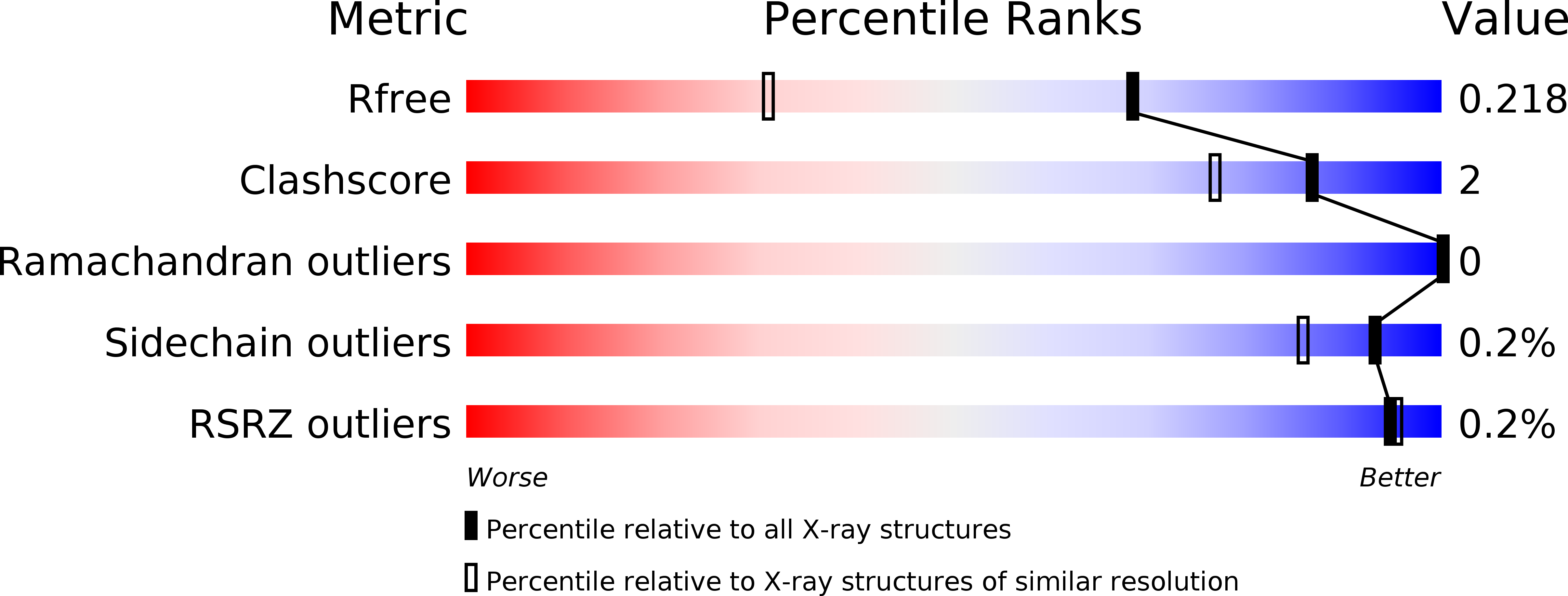
6XZE, 6XZO, 6XZS, 6XZX, 6XZY, 6Y00 - PubMed Abstract:
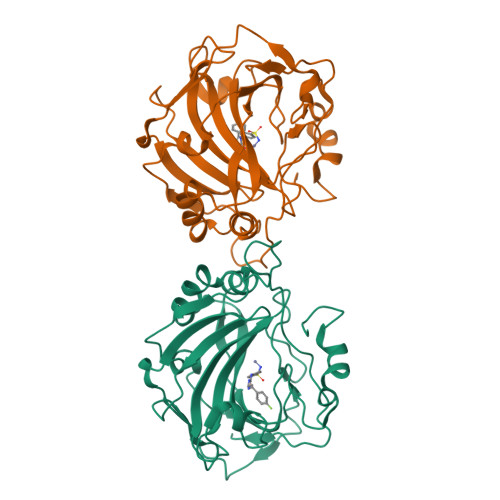
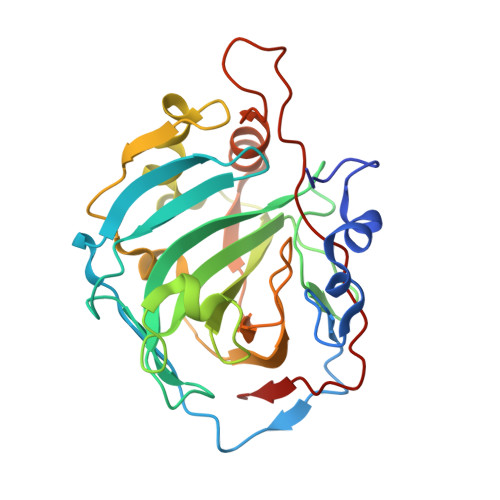
A drug design strategy of carbonic anhydrase inhibitors (CAIs) belonging to sulfonamides incorporating ureidoethylaminobenzyl tails is presented. A variety of substitution patterns on the ring and the tails, located on para - or meta - positions with respect to the sulfonamide warheads were incorporated in the new compounds. Inhibition of human carbonic anhydrases (hCA) isoforms I, II, IX and XII, involving various pathologies, was assessed with the new compounds. Selective inhibitory profile towards hCA II was observed, the most active compounds being low nM inhibitors ( K I s of 2.8-9.2 nM, respectively). Extensive X-ray crystallographic analysis of several sulfonamides in an adduct with hCA I allowed an in-depth understanding of their binding mode and to lay a detailed structure-activity relationship.
Organizational Affiliation:
Dipartimento Neurofarba, Università degli Studi di Firenze, Sezione di Scienze Farmaceutiche, Polo Scientifico, Via Ugo Schiff 6, Sesto Fiorentino, 50019 Florence, Italy.