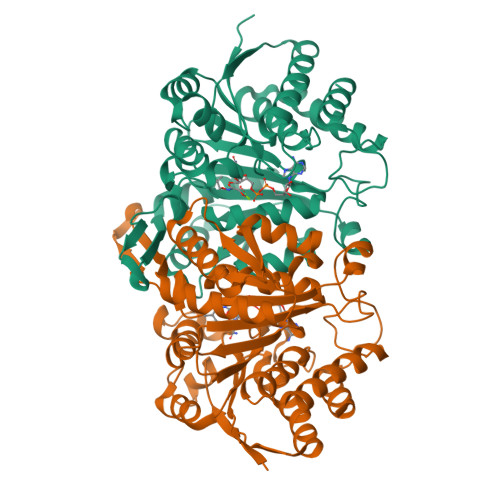
Crystal structure of Haemophilus influenzae 3-isopropylmalate dehydrogenase (LeuB) in complex with the inhibitor O-isobutenyl oxalylhydroxamate.
Miggiano, R., Martignon, S., Minassi, A., Rossi, F., Rizzi, M.(2020) Biochem Biophys Res Commun 524: 996-1002
- PubMed: 32059844
- DOI: https://doi.org/10.1016/j.bbrc.2020.02.022
- Primary Citation of Related Structures:
6XXY - PubMed Abstract:
3-isopropylmalate dehydrogenases (LeuB) belong to the leucine biosynthetic pathway and catalyze the irreversible oxidative decarboxylation of 3IPM to 2-ketoisocaproate that is finally converted into leucine by a branched-chain aminotransferase. Since leucine is an essential amino acid for humans, and it is also vital for the growth of many pathogenic bacteria, the enzymes belonging to this pathway can be considered as potential target sites for designing of a new class of antibacterial agents. We have determined the crystal structure of the Haemophilus influenzae LeuB in complex with the cofactor NAD + and the inhibitor O-IbOHA, at 2.1 Å resolution; moreover, we have investigated the inhibitor mechanism of action by analyzing the enzyme kinetics. The structure of H. influenzae LeuB in complex with the intermediate analog inhibitor displays a fully closed conformation, resembling the previously observed, closed form of the equivalent enzyme of Thiobacillus ferrooxidans in complex with the 3IPM substrate. O-IbOHA was found to bind the active site by adopting the same conformation of 3IPM, and to induce an unreported repositioning of the side chain of the amino acids that participate in the coordination of the ligand. Indeed, the experimentally observed binding mode of O-IbOHA to the H. influenzae LeuB enzyme, reveals aspects of novelty compared to the computational binding prediction performed on M. tuberculosis LeuB. Overall, our data provide new insights for the structure-based rational design of a new class of antibiotics targeting the biosynthesis of leucine in pathogenic bacteria.
Organizational Affiliation:
Department of Pharmaceutical Sciences, University of Piemonte Orientale, Via Bovio 6, 28100, Novara, Italy. Electronic address: riccardo.miggiano@uniupo.it.