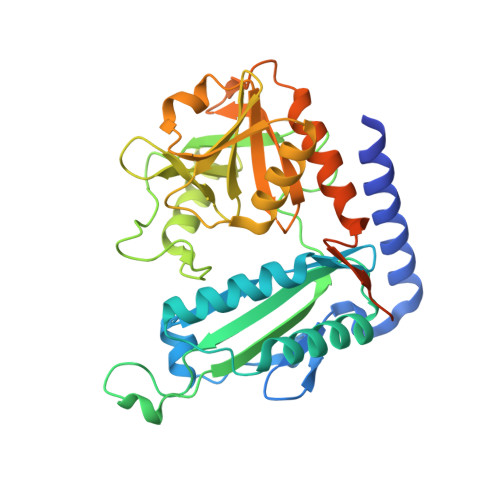
An ( R )-Selective Transaminase From Thermomyces stellatus : Stabilizing the Tetrameric Form.
Heckmann, C.M., Gourlay, L.J., Dominguez, B., Paradisi, F.(2020) Front Bioeng Biotechnol 8: 707-707
- PubMed: 32793563
- DOI: https://doi.org/10.3389/fbioe.2020.00707
- Primary Citation of Related Structures:
6XWB - PubMed Abstract:
The identification and 3D structural characterization of a homolog of the ( R )-selective transaminase (RTA) from Aspergillus terreus ( At RTA), from the thermotolerant fungus Thermomyces stellatus ( Ts RTA) is here reported. The thermostability of Ts RTA (40% retained activity after 7 days at 40°C) was initially attributed to its tetrameric form in solution, however subsequent studies of At RTA revealed it also exists predominantly as a tetramer yet, at 40°C, it is inactivated within 48 h. The engineering of a cysteine residue to promote disulfide bond formation across the dimer-dimer interface stabilized both enzymes, with Ts RTA_G205C retaining almost full activity after incubation at 50°C for 7 days. Thus, the role of this mutation was elucidated and the importance of stabilizing the tetramer for overall stability of RTAs is highlighted. Ts RTA accepts the common amine donors ( R )-methylbenzylamine, isopropylamine, and d-alanine as well as aromatic and aliphatic ketones and aldehydes.
Organizational Affiliation:
School of Chemistry, University of Nottingham, Nottingham, United Kingdom.