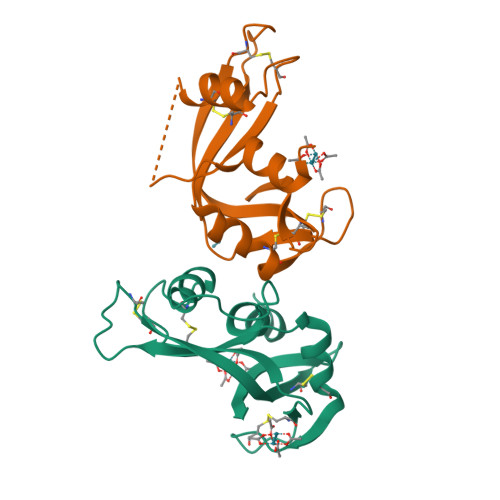
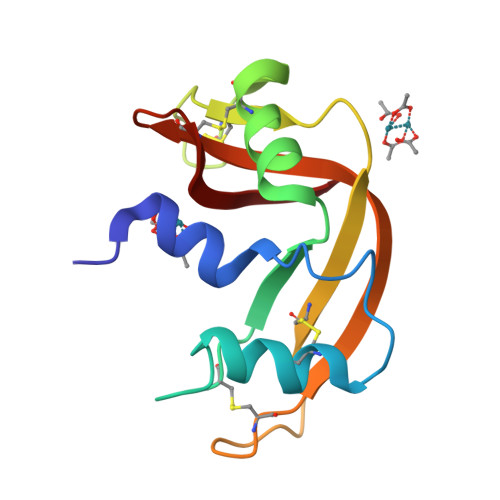
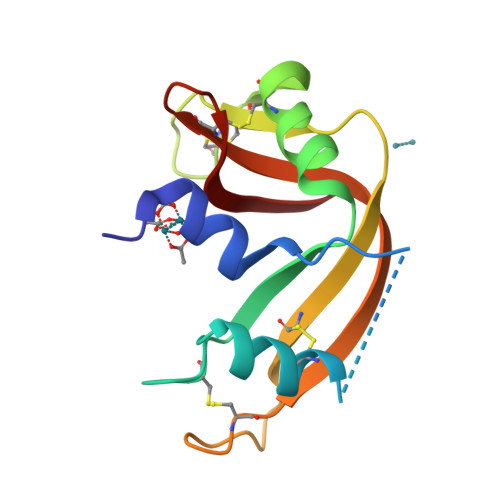
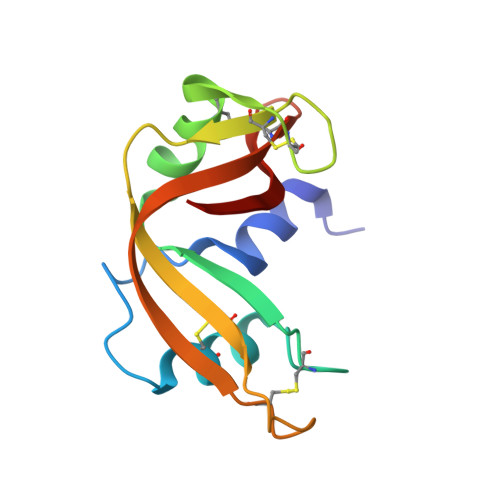
Protein interactions of dirhodium tetraacetate: a structural study.
Ferraro, G., Pratesi, A., Messori, L., Merlino, A.(2020) Dalton Trans 49: 2412-2416
- PubMed: 32022076
- DOI: https://doi.org/10.1039/c9dt04819g
- Primary Citation of Related Structures:
6XVX, 6XW0 - PubMed Abstract:
The interactions between the cytotoxic paddlewheel dirhodium complex [Rh2(μ-O2CCH3)4] and the model protein bovine pancreatic ribonuclease (RNase A) were investigated by high-resolution mass spectrometry and X-ray crystallography. The results indicate that [Rh2(μ-O2CCH3)4] extensively reacts with RNase A. The metal compound binds the protein via coordination of the imidazole ring of a His side chain to one of its axial sites, while the dirhodium center and the acetato ligands remain unmodified. Data provide valuable information for the design of artificial dirhodium-containing metalloenzymes.
Organizational Affiliation:
Department of Chemistry "Ugo Schiff", University of Florence, via della Lastruccia, 3-13, 50019, Sesto Fiorentino, Florence, Italy. luigi.messori@unifi.it.