Structure-based amelioration of PXR transactivation in a novel series of macrocyclic allosteric inhibitors of HIV-1 integrase.
Sivaprakasam, P., Wang, Z., Meanwell, N.A., Khan, J.A., Langley, D.R., Johnson, S.R., Li, G., Pendri, A., Connolly, T.P., Gao, M., Camac, D.M., Klakouski, C., Zvyaga, T., Cianci, C., McAuliffe, B., Ding, B., Discotto, L., Krystal, M.R., Jenkins, S., Peese, K.M., Narasimhulu Naidu, B.(2020) Bioorg Med Chem Lett 30: 127531-127531
- PubMed: 32890685
- DOI: https://doi.org/10.1016/j.bmcl.2020.127531
- Primary Citation of Related Structures:
6XP9 - PubMed Abstract:
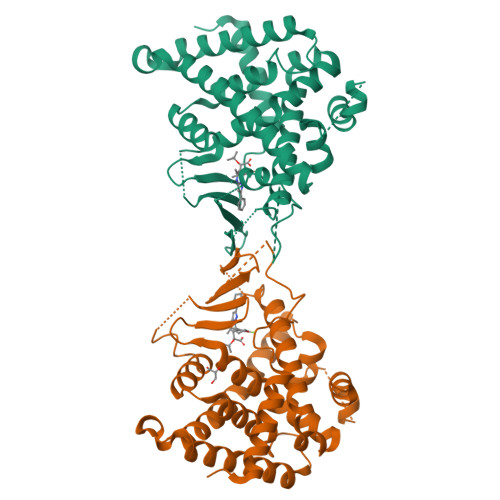
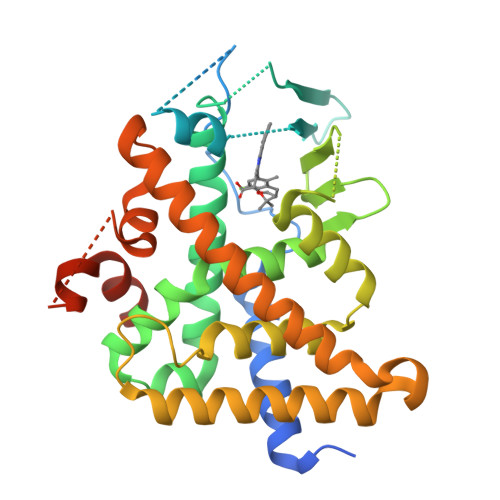
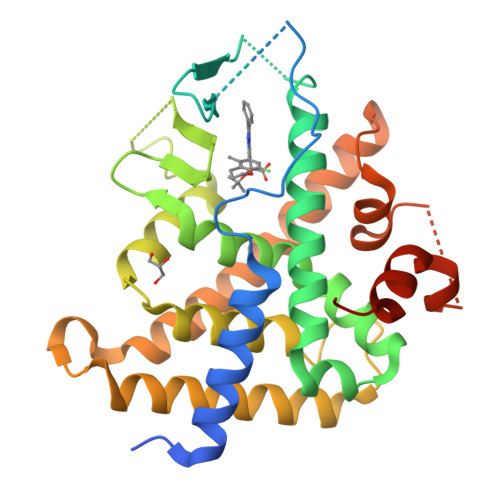
Previous studies have identified a series of imidazo[1,2-a]pyridine (IZP) derivatives as potent allosteric inhibitors of HIV-1 integrase (ALLINIs) and virus infection in cell culture. However, IZPs were also found to be relatively potent activators of the pregnane-X receptor (PXR), raising the specter of induction of CYP-mediated drug disposition pathways. In an attempt to modify PXR activity without affecting anti-HIV-1 activity, rational structure-based design and modeling approaches were used. An X-ray cocrystal structure of (S,S)-1 in the PXR ligand binding domain (LBD) allowed an examination of the potential of rational structural modifications designed to abrogate PXR. The introduction of bulky basic amines at the C-8 position provided macrocyclic IZP derivatives that displayed potent HIV-1 inhibitory activity in cell culture with no detectable PXR transactivation at the highest concentration tested.
Organizational Affiliation:
Computer-Aided Drug Design, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, USA. Electronic address: prasanna.siva@bms.co.