A trimethoprim derivative impedes antibiotic resistance evolution.
Manna, M.S., Tamer, Y.T., Gaszek, I., Poulides, N., Ahmed, A., Wang, X., Toprak, F.C.R., Woodard, D.R., Koh, A.Y., Williams, N.S., Borek, D., Atilgan, A.R., Hulleman, J.D., Atilgan, C., Tambar, U., Toprak, E.(2021) Nat Commun 12: 2949-2949
- PubMed: 34011959
- DOI: https://doi.org/10.1038/s41467-021-23191-z
- Primary Citation of Related Structures:
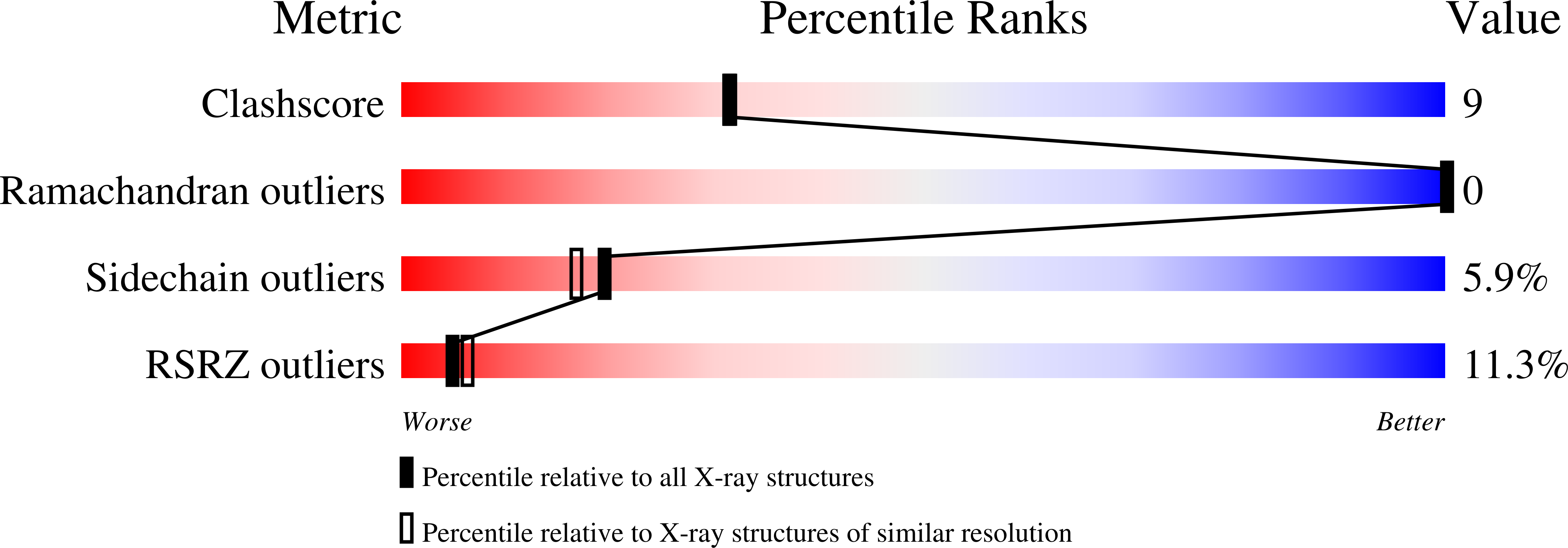
6XG4, 6XG5 - PubMed Abstract:
The antibiotic trimethoprim (TMP) is used to treat a variety of Escherichia coli infections, but its efficacy is limited by the rapid emergence of TMP-resistant bacteria. Previous laboratory evolution experiments have identified resistance-conferring mutations in the gene encoding the TMP target, bacterial dihydrofolate reductase (DHFR), in particular mutation L28R. Here, we show that 4'-desmethyltrimethoprim (4'-DTMP) inhibits both DHFR and its L28R variant, and selects against the emergence of TMP-resistant bacteria that carry the L28R mutation in laboratory experiments. Furthermore, antibiotic-sensitive E. coli populations acquire antibiotic resistance at a substantially slower rate when grown in the presence of 4'-DTMP than in the presence of TMP. We find that 4'-DTMP impedes evolution of resistance by selecting against resistant genotypes with the L28R mutation and diverting genetic trajectories to other resistance-conferring DHFR mutations with catalytic deficiencies. Our results demonstrate how a detailed characterization of resistance-conferring mutations in a target enzyme can help identify potential drugs against antibiotic-resistant bacteria, which may ultimately increase long-term efficacy of antimicrobial therapies by modulating evolutionary trajectories that lead to resistance.
Organizational Affiliation:
Green Center for Systems Biology, University of Texas Southwestern Medical Center, Dallas, TX, USA.