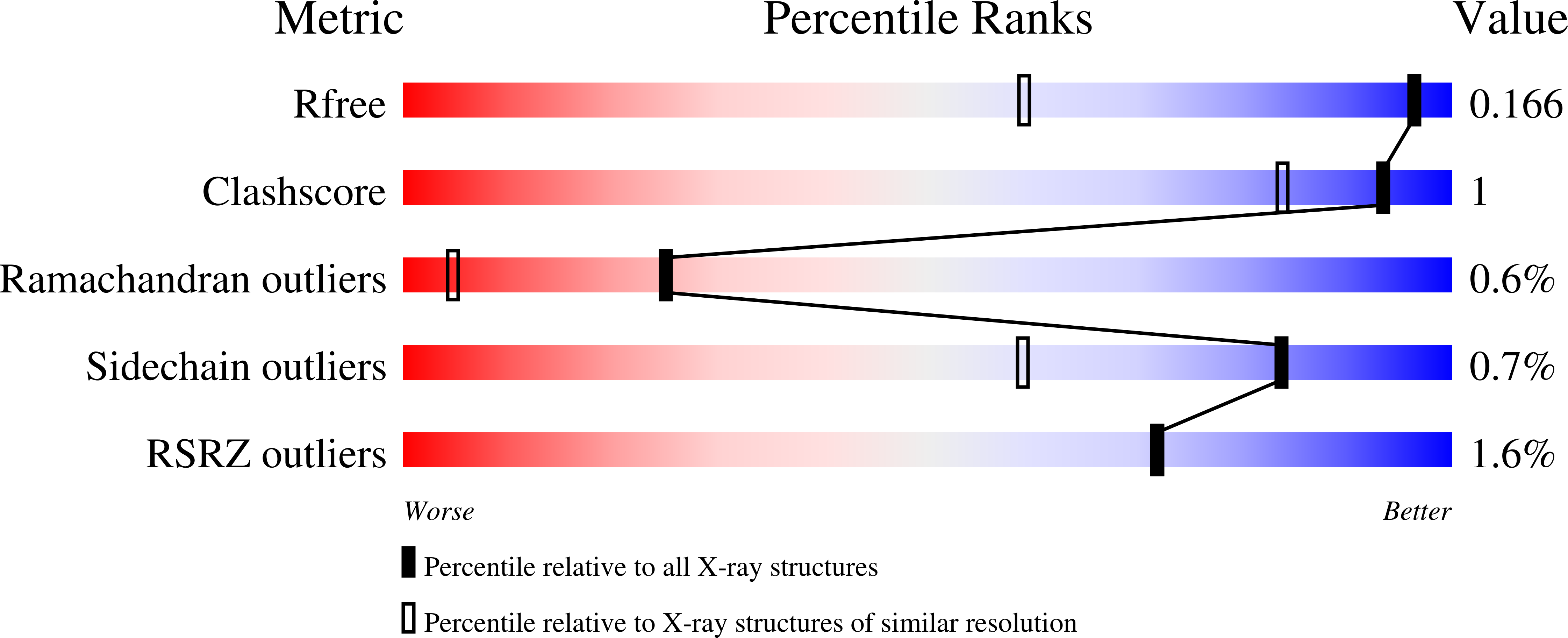
Structural Basis of Ligand Selectivity by a Bacterial Adhesin Lectin Involved in Multispecies Biofilm Formation.
Guo, S., Vance, T.D.R., Zahiri, H., Eves, R., Stevens, C., Hehemann, J.H., Vidal-Melgosa, S., Davies, P.L.(2021) mBio 12
- PubMed: 33824212
- DOI: https://doi.org/10.1128/mBio.00130-21
- Primary Citation of Related Structures:
6X7J, 6X7T, 6X7X, 6X7Y, 6X7Z, 6X8A, 6X8D, 6X8Y, 6X95, 6X9M, 6X9P, 6XA5, 6XAC, 6XAQ - PubMed Abstract:
Carbohydrate recognition by lectins governs critical host-microbe interactions. Mp PA14 ( Marinomonas primoryensis PA14 domain) lectin is a domain of a 1.5-MDa adhesin responsible for a symbiotic bacterium-diatom interaction in Antarctica. Here, we show that Mp PA14 binds various monosaccharides, with l-fucose and N -acetylglucosamine being the strongest ligands (dissociation constant [ K d ], ∼150 μM). High-resolution structures of Mp PA14 with 15 different sugars bound elucidated the molecular basis for the lectin's apparent binding promiscuity but underlying selectivity. Mp PA14 mediates strong Ca 2+ -dependent interactions with the 3,4-diols of l-fucopyranose and glucopyranoses, and it binds other sugars via their specific minor isomers. Thus, Mp PA14 only binds polysaccharides like branched glucans and fucoidans with these free end groups. Consistent with our findings, adhesion of Mp PA14 to diatom cells was selectively blocked by l-fucose, but not by N -acetyl galactosamine. The Mp PA14 lectin homolog present in a Vibrio cholerae adhesin was produced and was shown to have the same sugar binding preferences as Mp PA14. The pathogen's lectin was unable to effectively bind the diatom in the presence of fucose, thus demonstrating the antiadhesion strategy of blocking infection via ligand-based antagonists. IMPORTANCE Bacterial adhesins are key virulence factors that are essential for the pathogen-host interaction and biofilm formation that cause most infections. Many of the adhesin-driven cell-cell interactions are mediated by lectins. Our study reveals for the first time the molecular basis underlying the binding selectivity of a common bacterial adhesin lectin from the marine bacterium Marinomonas primoryensis , homologs of which are found in both environmental and pathogenic species. The lectin-ligand interactions illustrated at the atomic level guided the identification of a ligand that serves as an inhibitor to block bacterium-host adhesion. With conventional bactericidal antibiotics losing their potency due to resistance, our work gives critical insight into an antiadhesion strategy to treat bacterial infections.
Organizational Affiliation:
Department of Biomedical and Molecular Sciences, Queen's University, Kingston, Ontario, Canada.