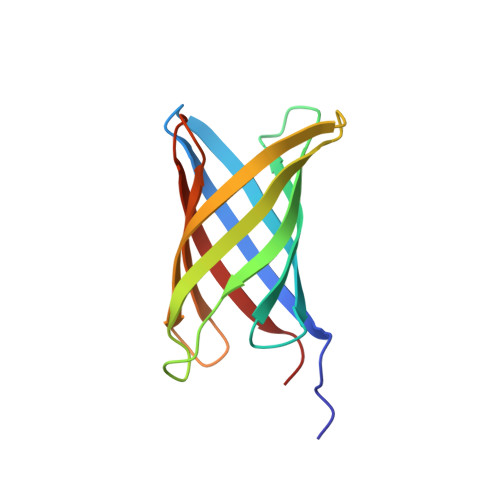
De novo design of transmembrane beta barrels.
Vorobieva, A.A., White, P., Liang, B., Horne, J.E., Bera, A.K., Chow, C.M., Gerben, S., Marx, S., Kang, A., Stiving, A.Q., Harvey, S.R., Marx, D.C., Khan, G.N., Fleming, K.G., Wysocki, V.H., Brockwell, D.J., Tamm, L.K., Radford, S.E., Baker, D.(2021) Science 371
- PubMed: 33602829
- DOI: https://doi.org/10.1126/science.abc8182
- Primary Citation of Related Structures:
6X1K, 6X9Z - PubMed Abstract:
Transmembrane β-barrel proteins (TMBs) are of great interest for single-molecule analytical technologies because they can spontaneously fold and insert into membranes and form stable pores, but the range of pore properties that can be achieved by repurposing natural TMBs is limited. We leverage the power of de novo computational design coupled with a "hypothesis, design, and test" approach to determine TMB design principles, notably, the importance of negative design to slow β-sheet assembly. We design new eight-stranded TMBs, with no homology to known TMBs, that insert and fold reversibly into synthetic lipid membranes and have nuclear magnetic resonance and x-ray crystal structures very similar to the computational models. These advances should enable the custom design of pores for a wide range of applications.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195, USA.