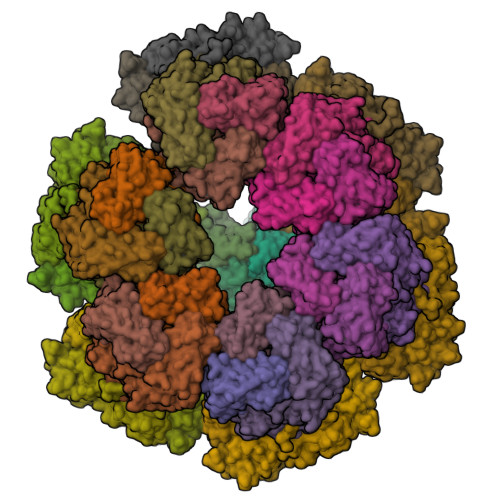
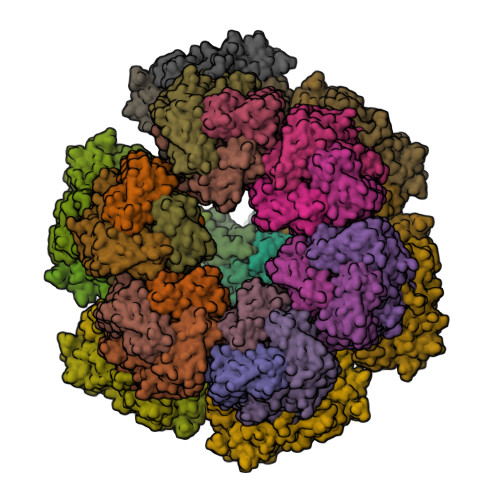
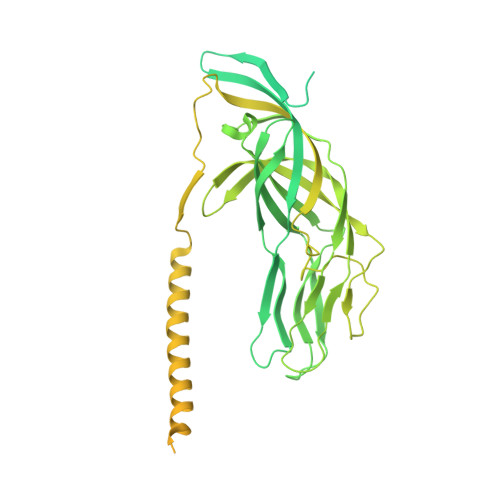
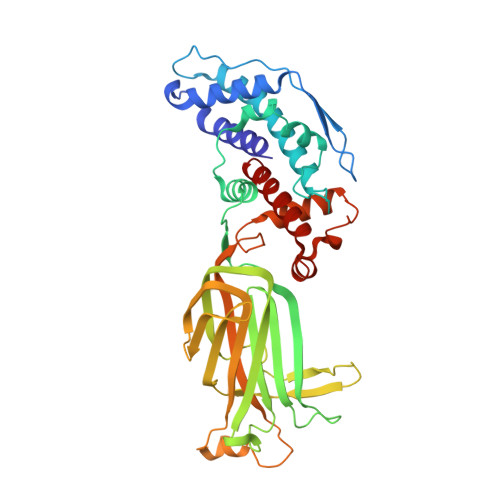
Functional refolding of the penetration protein on a non-enveloped virus.
Herrmann, T., Torres, R., Salgado, E.N., Berciu, C., Stoddard, D., Nicastro, D., Jenni, S., Harrison, S.C.(2021) Nature 590: 666-670
- PubMed: 33442061
- DOI: https://doi.org/10.1038/s41586-020-03124-4
- Primary Citation of Related Structures:
6WXE, 6WXF, 6WXG - PubMed Abstract:
A non-enveloped virus requires a membrane lesion to deliver its genome into a target cell 1 . For rotaviruses, membrane perforation is a principal function of the viral outer-layer protein, VP4 2,3 . Here we describe the use of electron cryomicroscopy to determine how VP4 performs this function and show that when activated by cleavage to VP8* and VP5*, VP4 can rearrange on the virion surface from an 'upright' to a 'reversed' conformation. The reversed structure projects a previously buried 'foot' domain outwards into the membrane of the host cell to which the virion has attached. Electron cryotomograms of virus particles entering cells are consistent with this picture. Using a disulfide mutant of VP4, we have also stabilized a probable intermediate in the transition between the two conformations. Our results define molecular mechanisms for the first steps of the penetration of rotaviruses into the membranes of target cells and suggest similarities with mechanisms postulated for other viruses.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA, USA.