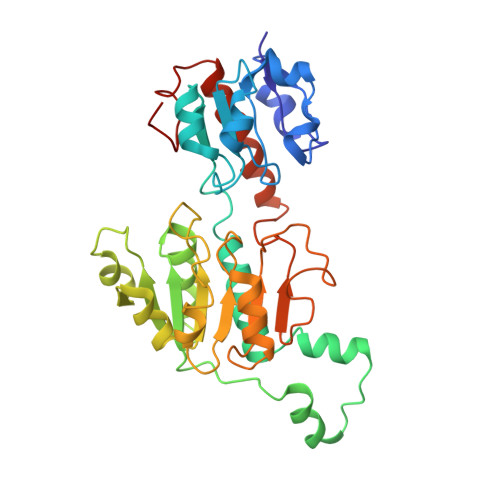
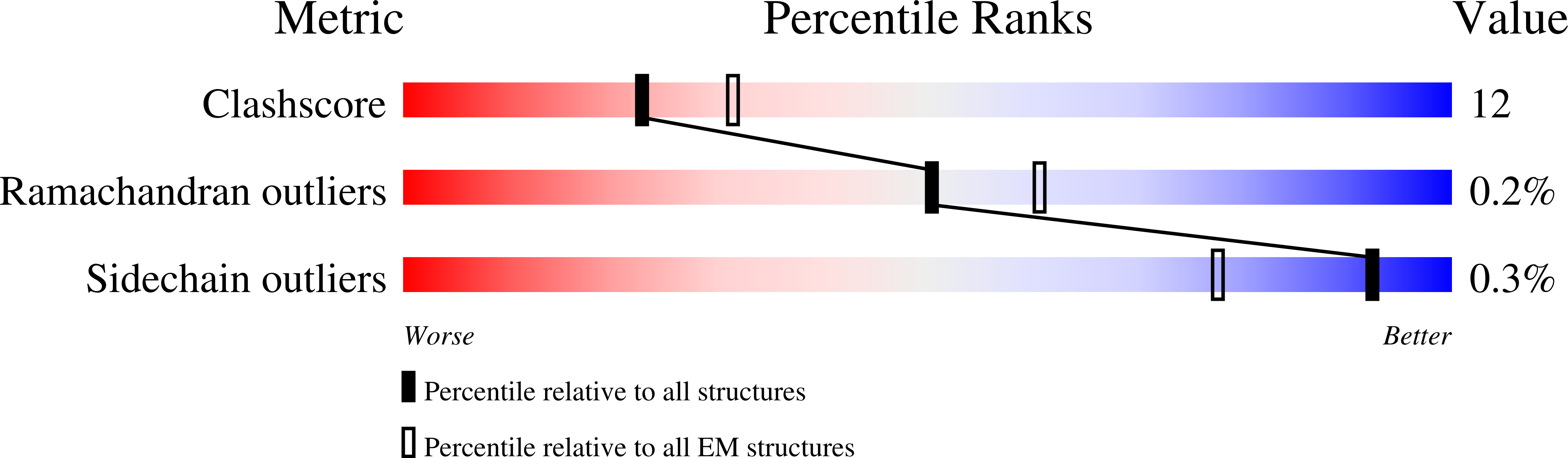Cryo-EM structure of CtBP2 confirms tetrameric architecture.
Jecrois, A.M., Dcona, M.M., Deng, X., Bandyopadhyay, D., Grossman, S.R., Schiffer, C.A., Royer Jr., W.E.(2021) Structure 29: 310
- PubMed: 33264605
- DOI: https://doi.org/10.1016/j.str.2020.11.008
- Primary Citation of Related Structures:
6WKW - PubMed Abstract:
C-terminal binding proteins 1 and 2 (CtBP1 and CtBP2) are transcriptional regulators that activate or repress many genes involved in cellular development, apoptosis, and metastasis. NADH-dependent CtBP activation has been implicated in multiple types of cancer and poor patient prognosis. Central to understanding activation of CtBP in oncogenesis is uncovering how NADH triggers protein assembly, what level of assembly occurs, and if oncogenic activity depends upon such assembly. Here, we present the cryoelectron microscopic structures of two different constructs of CtBP2 corroborating that the native state of CtBP2 in the presence of NADH is tetrameric. The physiological relevance of the observed tetramer was demonstrated in cell culture, showing that CtBP tetramer-destabilizing mutants are defective for cell migration, transcriptional repression of E-cadherin, and activation of TIAM1. Together with our cryoelectron microscopy studies, these results highlight the tetramer as the functional oligomeric form of CtBP2.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester, MA 01605, USA.