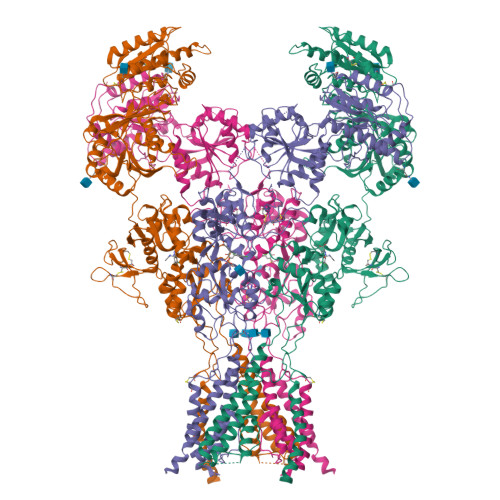
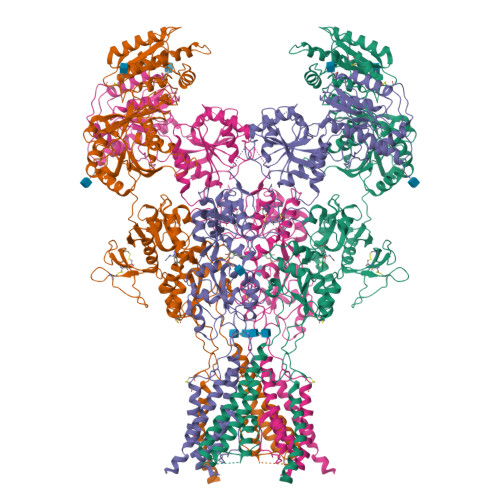
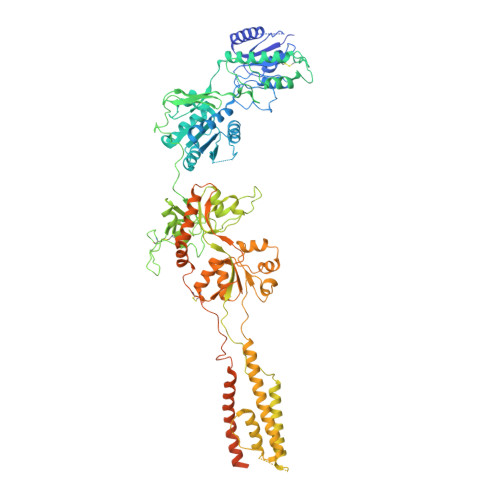
Structural Basis of Functional Transitions in Mammalian NMDA Receptors.
Chou, T.H., Tajima, N., Romero-Hernandez, A., Furukawa, H.(2020) Cell 182: 357-371.e13
- PubMed: 32610085
- DOI: https://doi.org/10.1016/j.cell.2020.05.052
- Primary Citation of Related Structures:
6USU, 6USV, 6WHR, 6WHS, 6WHT, 6WHU, 6WHV, 6WHW, 6WHX, 6WHY, 6WI0, 6WI1 - PubMed Abstract:
Excitatory neurotransmission meditated by glutamate receptors including N-methyl-D-aspartate receptors (NMDARs) is pivotal to brain development and function. NMDARs are heterotetramers composed of GluN1 and GluN2 subunits, which bind glycine and glutamate, respectively, to activate their ion channels. Despite importance in brain physiology, the precise mechanisms by which activation and inhibition occur via subunit-specific binding of agonists and antagonists remain largely unknown. Here, we show the detailed patterns of conformational changes and inter-subunit and -domain reorientation leading to agonist-gating and subunit-dependent competitive inhibition by providing multiple structures in distinct ligand states at 4 Å or better. The structures reveal that activation and competitive inhibition by both GluN1 and GluN2 antagonists occur by controlling the tension of the linker between the ligand-binding domain and the transmembrane ion channel of the GluN2 subunit. Our results provide detailed mechanistic insights into NMDAR pharmacology, activation, and inhibition, which are fundamental to the brain physiology.
Organizational Affiliation:
WM Keck Structural Biology Laboratory, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA.