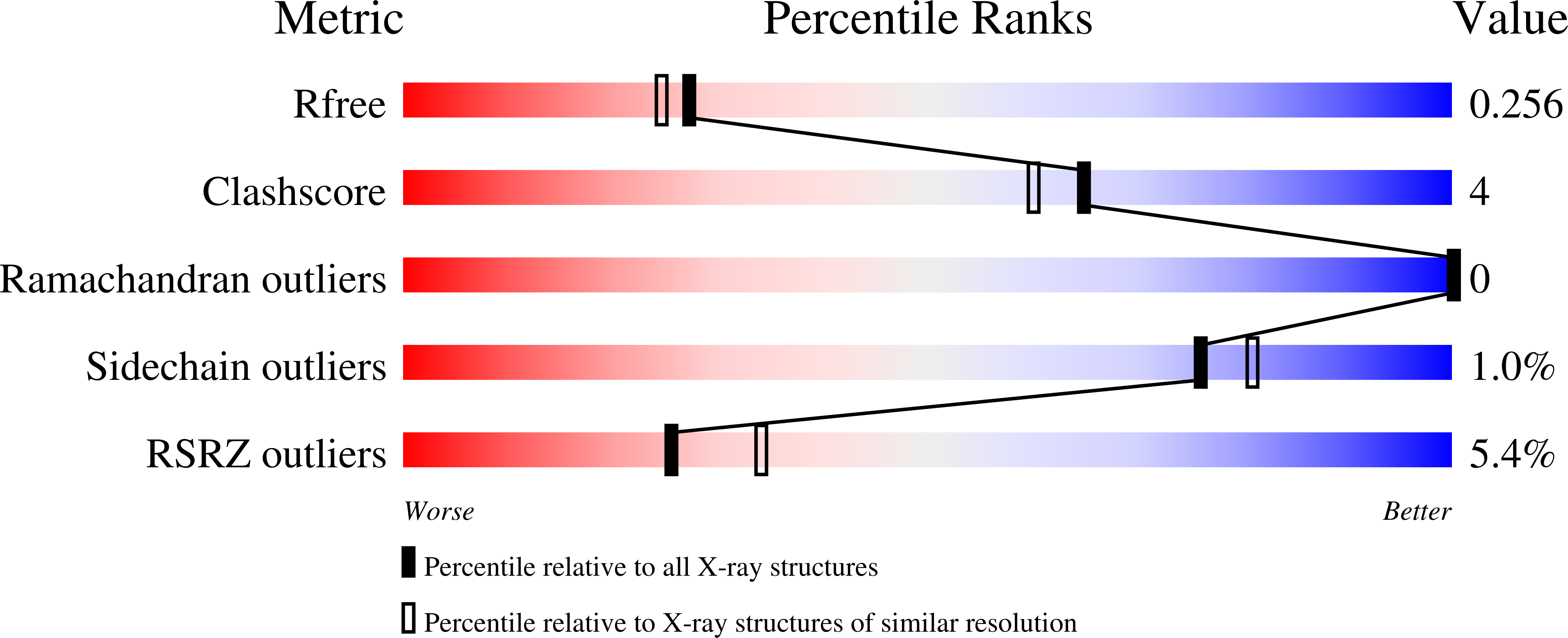
Proton Relay Network in the Bacterial P450s: CYP101A1 and CYP101D1.
Amaya, J.A., Batabyal, D., Poulos, T.L.(2020) Biochemistry 59: 2896-2902
- PubMed: 32574066
- DOI: https://doi.org/10.1021/acs.biochem.0c00329
- Primary Citation of Related Structures:
6WE6, 6WFL, 6WGW - PubMed Abstract:
Cytochrome P450s are among nature's most powerful catalysts. Their ability to activate molecular dioxygen to form high-valent ferryl intermediates (Compounds I and II) enables a wide array of chemistries ranging from simple epoxidations to more complicated C-H bond oxidations. Oxygen activation is achieved by reduction of the ferrous dioxygen complex, which requires the transfer of an electron from a redox partner and subsequent double protonation to yield a water molecule and a ferryl porphyrin π-cation radical (Compound I). Previous studies of the CYP101 family of cytochrome P450s demonstrated the importance of the conserved active site Asp25X residue in this protonation event, although its precise role is yet to be unraveled. To further explore the origin of protons in oxygen activation, we analyzed the effects of an Asp to Glu mutation at the 25X position in P450cam and in CYP101D1. This mutation inactivates P450cam but not CYP101D1. A series of mutagenic, crystallographic, kinetic, and molecular dynamics studies indicate that this mutation locks P450cam into a closed, inactive conformation. In CYP101D1, the D259E mutant changes the rate-limiting step to reduction of the P450-oxy complex, thus opening a window into the critical proton-coupled electron transfer step in P450 catalysis.
Organizational Affiliation:
Departments of Molecular Biology and Biochemistry, Pharmaceutical Sciences, and Chemistry, University of California, Irvine, California 92697-3900, United States.