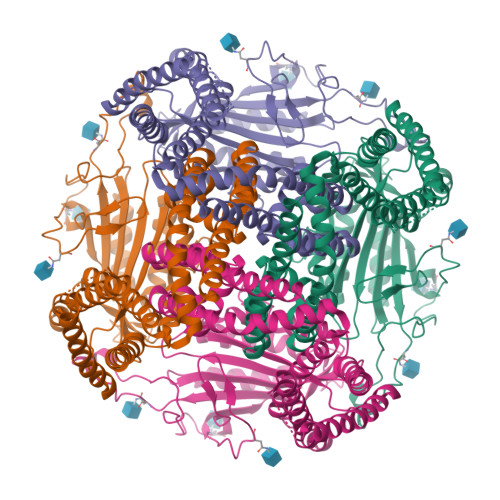
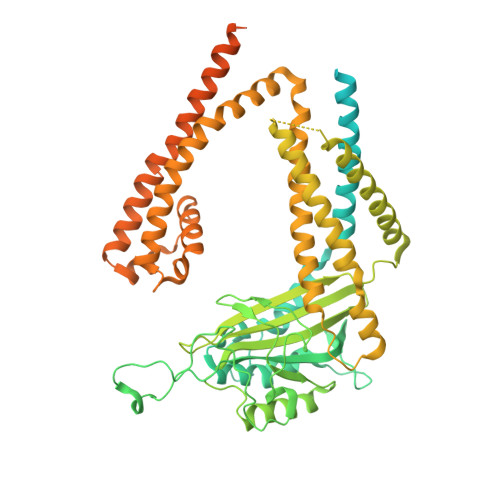
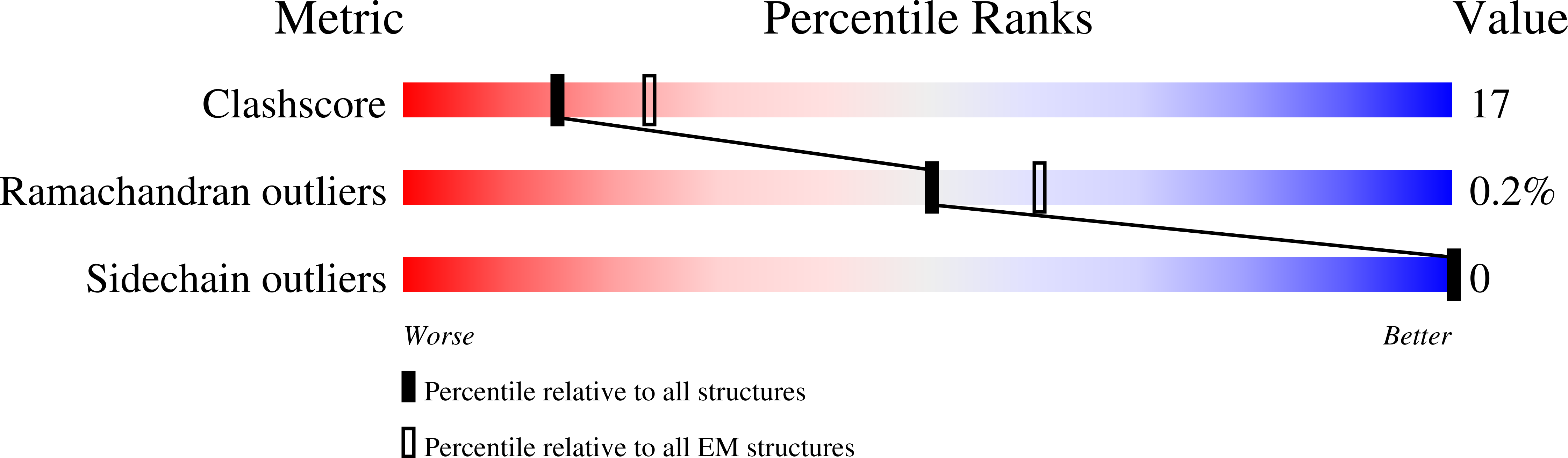Molecular dysregulation of ciliary polycystin-2 channels caused by variants in the TOP domain.
Vien, T.N., Wang, J., Ng, L.C.T., Cao, E., DeCaen, P.G.(2020) Proc Natl Acad Sci U S A 117: 10329-10338
- PubMed: 32332171
- DOI: https://doi.org/10.1073/pnas.1920777117
- Primary Citation of Related Structures:
6WB8 - PubMed Abstract:
Genetic variants in PKD2 which encodes for the polycystin-2 ion channel are responsible for many clinical cases of autosomal dominant polycystic kidney disease (ADPKD). Despite our strong understanding of the genetic basis of ADPKD, we do not know how most variants impact channel function. Polycystin-2 is found in organelle membranes, including the primary cilium-an antennae-like structure on the luminal side of the collecting duct. In this study, we focus on the structural and mechanistic regulation of polycystin-2 by its TOP domain-a site with unknown function that is commonly altered by missense variants. We use direct cilia electrophysiology, cryogenic electron microscopy, and superresolution imaging to determine that variants of the TOP domain finger 1 motif destabilizes the channel structure and impairs channel opening without altering cilia localization and channel assembly. Our findings support the channelopathy classification of PKD2 variants associated with ADPKD, where polycystin-2 channel dysregulation in the primary cilia may contribute to cystogenesis.
Organizational Affiliation:
Department of Pharmacology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611.