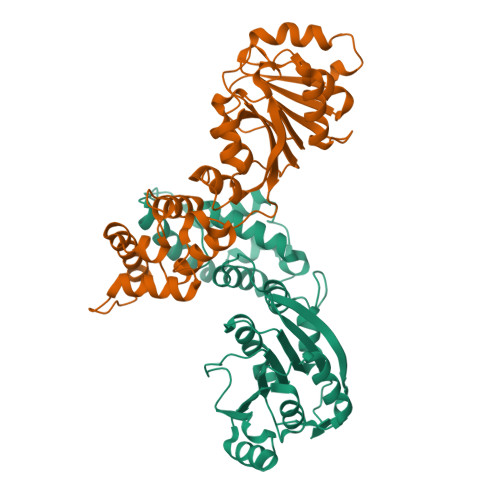
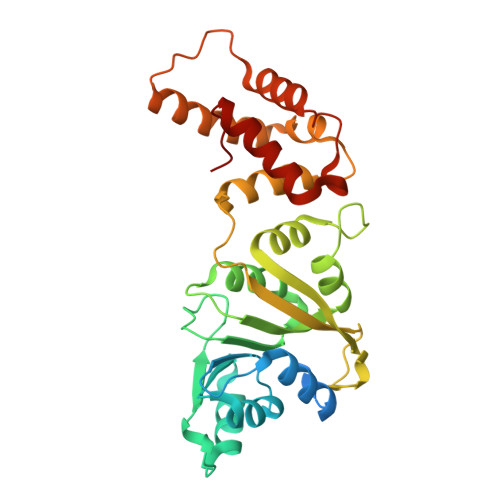
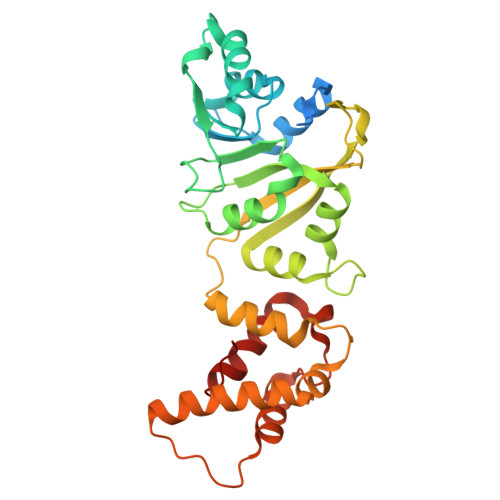
Structural and catalytic roles of the human 18SrRNA methyltransferases DIMT1 in ribosome assembly and translation.
Shen, H., Stoute, J., Liu, K.F.(2020) J Biological Chem 295: 12058-12070
- PubMed: 32616653
- DOI: https://doi.org/10.1074/jbc.RA120.014236
- Primary Citation of Related Structures:
6W6C, 6W6F - PubMed Abstract:
rRNA-modifying enzymes participate in ribosome assembly. However, whether the catalytic activities of these enzymes are important for the ribosome assembly and other cellular processes is not fully understood. Here, we report the crystal structure of WT human dimethyladenosine transferase 1 (DIMT1), an 18 S rRNA N 6,6 -dimethyladenosine (m 2 6,6 A) methyltransferase, and results obtained with a catalytically inactive DIMT1 variant. We found that DIMT1 +/- heterozygous HEK 293T cells have a significantly decreased 40S fraction and reduced protein synthesis but no major changes in m 2 6,6 A levels in 18 S rRNA. Expression of a catalytically inactive variant, DIMT1-E85A, in WT and DIMT1 +/- cells significantly decreased m 2 6,6 A levels in 18S rRNA, indicating a dominant-negative effect of this variant on m 2 6,6 A levels. However, expression of the DIMT1-E85A variant restored the defects in 40S levels. Of note, unlike WT DIMT1, DIMT1-E85A could not revert the defects in protein translation. We found that the differences between this variant and the WT enzyme extended to translation fidelity and gene expression patterns in DNA damage response pathways. These results suggest that the catalytic activity of DIMT1 is involved in protein translation and that the overall protein scaffold of DIMT1, regardless of the catalytic activity on m 2 6,6 A in 18 S rRNA, is essential for 40S assembly.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.