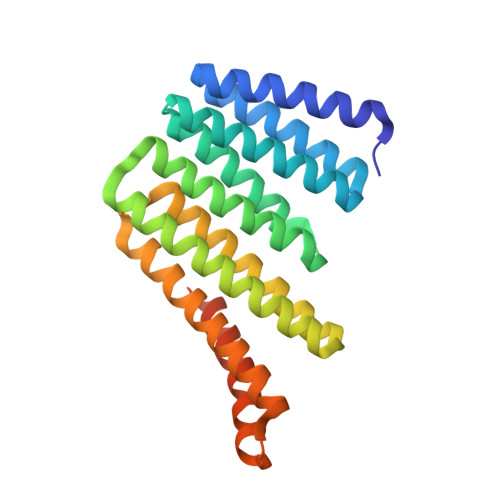
Modular repeat protein sculpting using rigid helical junctions.
Brunette, T.J., Bick, M.J., Hansen, J.M., Chow, C.M., Kollman, J.M., Baker, D.(2020) Proc Natl Acad Sci U S A 117: 8870-8875
- PubMed: 32245816
- DOI: https://doi.org/10.1073/pnas.1908768117
- Primary Citation of Related Structures:
6W2Q, 6W2R, 6W2V, 6W2W - PubMed Abstract:
The ability to precisely design large proteins with diverse shapes would enable applications ranging from the design of protein binders that wrap around their target to the positioning of multiple functional sites in specified orientations. We describe a protein backbone design method for generating a wide range of rigid fusions between helix-containing proteins and use it to design 75,000 structurally unique junctions between monomeric and homo-oligomeric de novo designed and ankyrin repeat proteins (RPs). Of the junction designs that were experimentally characterized, 82% have circular dichroism and solution small-angle X-ray scattering profiles consistent with the design models and are stable at 95 °C. Crystal structures of four designed junctions were in close agreement with the design models with rmsds ranging from 0.9 to 1.6 Å. Electron microscopic images of extended tetrameric structures and ∼10-nm-diameter "L" and "V" shapes generated using the junctions are close to the design models, demonstrating the control the rigid junctions provide for protein shape sculpting over multiple nanometer length scales.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195; tjbrunette@gmail.com.