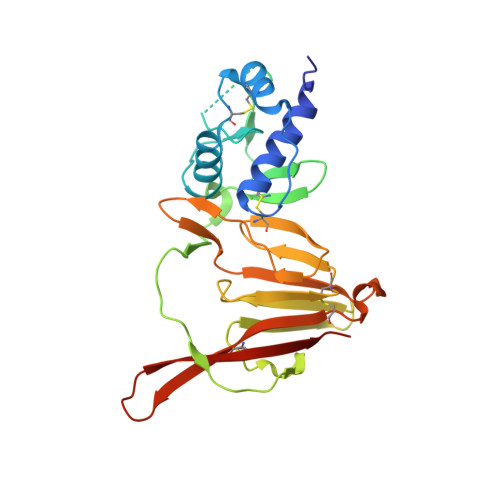
Structure and Characterization of Crimean-Congo Hemorrhagic Fever Virus GP38.
Mishra, A.K., Moyer, C.L., Abelson, D.M., Deer, D.J., El Omari, K., Duman, R., Lobel, L., Lutwama, J.J., Dye, J.M., Wagner, A., Chandran, K., Cross, R.W., Geisbert, T.W., Zeitlin, L., Bornholdt, Z.A., McLellan, J.S.(2020) J Virol 94
- PubMed: 31996434
- DOI: https://doi.org/10.1128/JVI.02005-19
- Primary Citation of Related Structures:
6VKF - PubMed Abstract:
Crimean-Congo hemorrhagic fever virus (CCHFV) is the causative agent of the most widespread tick-borne viral infection in humans. CCHFV encodes a secreted glycoprotein (GP38) of unknown function that is the target of a protective antibody. Here, we present the crystal structure of GP38 at a resolution of 2.5 Å, which revealed a novel fold primarily consisting of a 3-helix bundle and a β-sandwich. Sequence alignment and homology modeling showed distant homology between GP38 and the ectodomain of Gn (a structural glycoprotein in CCHFV), suggestive of a gene duplication event. Analysis of convalescent-phase sera showed high titers of GP38 antibodies indicating immunogenicity in humans during natural CCHFV infection. The only protective antibody for CCHFV in an adult mouse model reported to date, 13G8, bound GP38 with subnanomolar affinity and protected against heterologous CCHFV challenge in a STAT1-knockout mouse model. Our data strongly suggest that GP38 should be evaluated as a vaccine antigen and that its structure provides a foundation to investigate functions of this protein in the viral life cycle. IMPORTANCE Crimean-Congo hemorrhagic fever virus (CCHFV) is a priority pathogen that poses a high risk to public health. Due to the high morbidity and mortality rates associated with CCHFV infection, there is an urgent need to develop medical countermeasures for disease prevention and treatment. CCHFV GP38, a secreted glycoprotein of unknown function unique to the Nairoviridae family, was recently shown to be the target of a protective antibody against CCHFV. Here, we present the crystal structure of GP38, which revealed a novel fold with distant homology to another CCHFV glycoprotein that is suggestive of a gene duplication event. We also demonstrate that antibody 13G8 protects STAT1-knockout mice against heterologous CCHFV challenge using a clinical isolate from regions where CCHFV is endemic. Collectively, these data advance our understanding of GP38 structure and antigenicity and should facilitate future studies investigating its function.
Organizational Affiliation:
Department of Molecular Biosciences, University of Texas at Austin, Austin, Texas, USA.