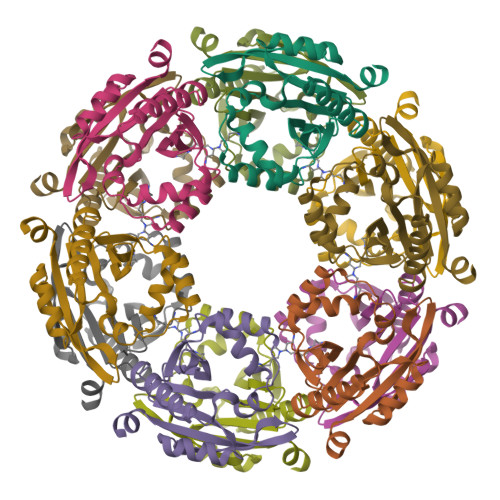
SpeG polyamine acetyltransferase enzyme from Bacillus thuringiensis forms a dodecameric structure and exhibits high catalytic efficiency.
Tsimbalyuk, S., Shornikov, A., Thi Bich Le, V., Kuhn, M.L., Forwood, J.K.(2020) J Struct Biol 210: 107506-107506
- PubMed: 32283314
- DOI: https://doi.org/10.1016/j.jsb.2020.107506
- Primary Citation of Related Structures:
6VFM, 6VFN - PubMed Abstract:
Polyamines are important for regulating biofilms and the exopolysaccharide of the biofilm matrix of Bacillus subtilis. Understanding how enzymes can regulate polyamine concentrations is critical for learning more about how these processes occur in diverse bacteria. Here, we describe the structure and function of another member of the spermidine/spermine acetyltransferases (SSAT) found in Bacilli. The SpeG enzyme from B. thuringiensis (BtSpeG) binds polyamines in its allosteric site and adopts a dodecameric oligomeric state similar to other SpeG enzymes from Gram-negative bacteria. Our kinetic results show the catalytic efficiency of BtSpeG was greater than any previously characterized SpeG to date, and in contrast to other SpeG proteins it exhibited very similar kinetic properties toward both spermine and spermidine. Similar to the SpeG enzyme from E. coli, BtSpeG was able to acetylate spermidine on the N 1 and N 8 positions. The turnover of BtSpeG toward spermine and spermidine was also two to three orders of magnitude greater than any other Bacilli SSAT enzyme that has been previously characterized. SpeG proteins from Bacilli, including B. cereus, B. thuringiensis and B. anthracis share nearly identical sequences and therefore our results likely provide insight into the structure/function relationship across multiple Bacillus species.
Organizational Affiliation:
School of Biomedical Sciences, Charles Sturt University, Wagga Wagga, NSW 2678, Australia.