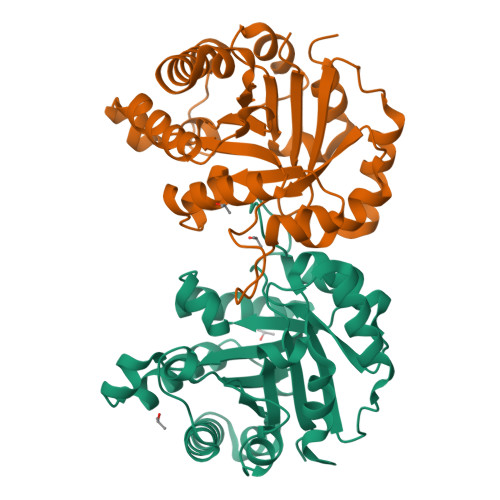
Triosephosphate isomerase deficiency: Effect of F240L mutation on enzyme structure.
Romero, J.M.(2020) Arch Biochem Biophys 689: 108473-108473
- PubMed: 32585311
- DOI: https://doi.org/10.1016/j.abb.2020.108473
- Primary Citation of Related Structures:
6UP1, 6UP5, 6UP8, 6UPF - PubMed Abstract:
Eleven missense mutations have been describe in human triosephosphate isomerase (TPI), affecting its catalytic function. Several of these mutations generate triosephosphate isomerase deficiency, the consequences of which can in some cases be lethal. The missense F240L mutation was found in a Hungarian patient showing symptoms of chronic hemolytic anemia and neuromuscular dysfunction. In vitro studies using a recombinant version of this mutant showed that it affects kinetic parameters, thermal stability and dimeric stability. Using X-ray crystal structures, the present paper describes how this mutation affected the flexibility of catalytic residues K13 and part of the (β/α) 8-barrel fold facing the dimeric interface in the TPI.
Organizational Affiliation:
Centro de Investigaciones en Química Biológica de Córdoba (CIQUIBIC), Universidad Nacional de Córdoba - Consejo Nacional de Investigaciones Científicas y Técnicas (UNC-CONICET), Departamento de Química Biológica Ranwel Caputto, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Haya de la Torre s/n, X5000HUA, Córdoba, Pabellón Argentina Ala Oeste, Argentina. Electronic address: jromero@fcq.unc.edu.ar.