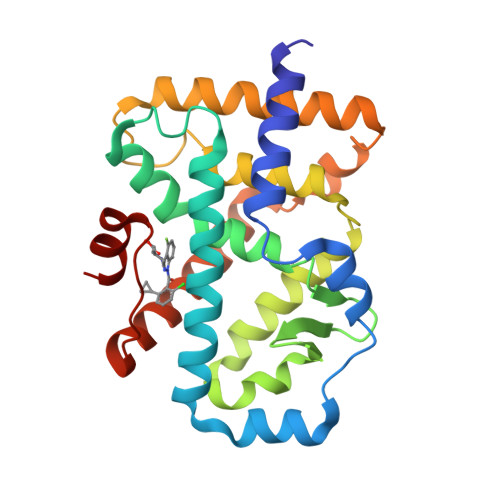
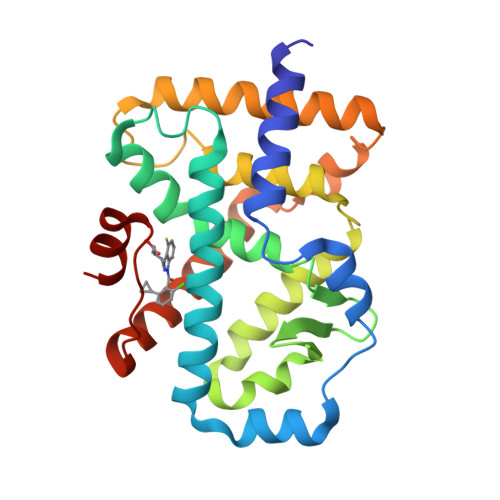
Discovery ofN-(Indazol-3-yl)piperidine-4-carboxylic Acids as ROR gamma t Allosteric Inhibitors for Autoimmune Diseases.
Zhang, H., Lapointe, B.T., Anthony, N., Azevedo, R., Cals, J., Correll, C.C., Daniels, M., Deshmukh, S., van Eenenaam, H., Ferguson, H., Hegde, L.G., Karstens, W.J., Maclean, J., Miller, J.R., Moy, L.Y., Simov, V., Nagpal, S., Oubrie, A., Palte, R.L., Parthasarathy, G., Sciammetta, N., van der Stelt, M., Woodhouse, J.D., Trotter, B.W., Barr, K.(2020) ACS Med Chem Lett 11: 114-119
- PubMed: 32071676
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00431
- Primary Citation of Related Structures:
6UCG - PubMed Abstract:
The clinical success of anti-IL-17 monoclonal antibodies (i.e., Cosentyx and Taltz) has validated Th17 pathway modulation for the treatment of autoimmune diseases. The nuclear hormone receptor RORγt is a master regulator of Th17 cells and affects the production of a host of cytokines, including IL-17A, IL-17F, IL-22, IL-26, and GM-CSF. Substantial interest has been spurred across both academia and industry to seek small molecules suitable for RORγt inhibition. A variety of RORγt inhibitors have been reported in the past few years, the majority of which are orthosteric binders. Here we disclose the discovery and optimization of a class of inhibitors, which bind differently to an allosteric binding pocket. Starting from a weakly active hit 1 , a tool compound 14 was quickly identified that demonstrated superior potency, selectivity, and off-target profile. Further optimization focused on improving metabolic stability. Replacing the benzoic acid moiety with piperidinyl carboxylate, modifying the 4-aza-indazole core in 14 to 4-F-indazole, and incorporating a key hydroxyl group led to the discovery of 25 , which possesses exquisite potency and selectivity, as well as an improved pharmacokinetic profile suitable for oral dosing.
Organizational Affiliation:
Medicinal Chemistry, Discovery Biology, Discovery Process Chemistry, Pharmacokinetics, Pharmacodynamics and Drug Metabolism, Discovery Pharmaceutical Sciences, Modeling & Informatics, In Vitro and In Vivo Pharmacology, and Computational and Structural Chemistry, Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, Massachusetts 02115, United States.