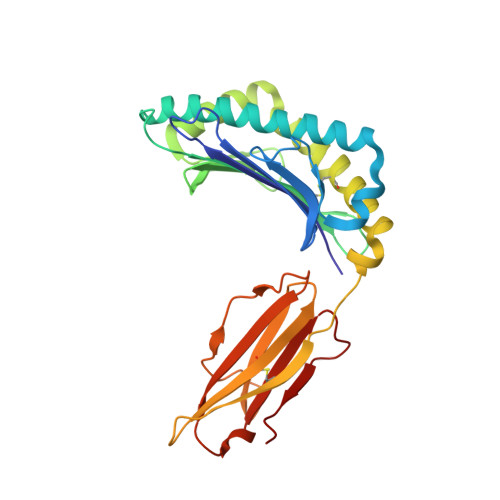
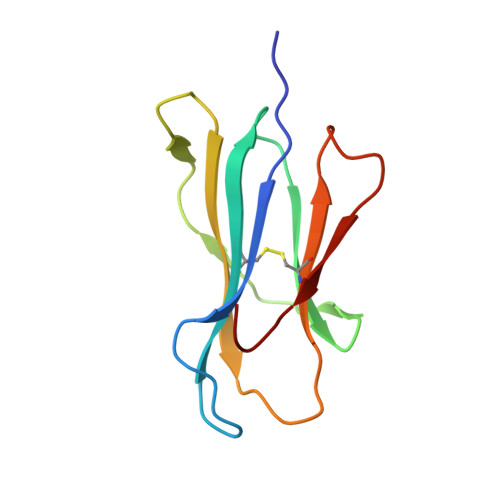
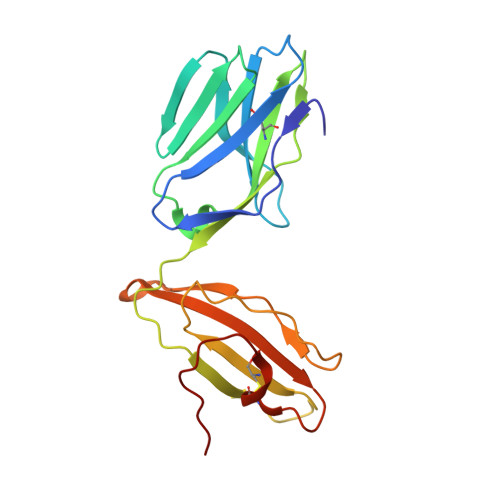
Molecular Rules Underpinning Enhanced Affinity Binding of Human T Cell Receptors Engineered for Immunotherapy.
Crean, R.M., MacLachlan, B.J., Madura, F., Whalley, T., Rizkallah, P.J., Holland, C.J., McMurran, C., Harper, S., Godkin, A., Sewell, A.K., Pudney, C.R., van der Kamp, M.W., Cole, D.K.(2020) Mol Ther Oncolytics 18: 443-456
- PubMed: 32913893
- DOI: https://doi.org/10.1016/j.omto.2020.07.008
- Primary Citation of Related Structures:
6TMO - PubMed Abstract:
Immuno-oncology approaches that utilize T cell receptors (TCRs) are becoming highly attractive because of their potential to target virtually all cellular proteins, including cancer-specific epitopes, via the recognition of peptide-human leukocyte antigen (pHLA) complexes presented at the cell surface. However, because natural TCRs generally recognize cancer-derived pHLAs with very weak affinities, efforts have been made to enhance their binding strength, in some cases by several million-fold. In this study, we investigated the mechanisms underpinning human TCR affinity enhancement by comparing the crystal structures of engineered enhanced affinity TCRs with those of their wild-type progenitors. Additionally, we performed molecular dynamics simulations to better understand the energetic mechanisms driving the affinity enhancements. These data demonstrate that supra-physiological binding affinities can be achieved without altering native TCR-pHLA binding modes via relatively subtle modifications to the interface contacts, often driven through the addition of buried hydrophobic residues. Individual energetic components of the TCR-pHLA interaction governing affinity enhancements were distinct and highly variable for each TCR, often resulting from additive, or knock-on, effects beyond the mutated residues. This comprehensive analysis of affinity-enhanced TCRs has important implications for the future rational design of engineered TCRs as efficacious and safe drugs for cancer treatment.
Organizational Affiliation:
Department of Biology and Biochemistry, University of Bath, Bath, BA2 7AY, UK.