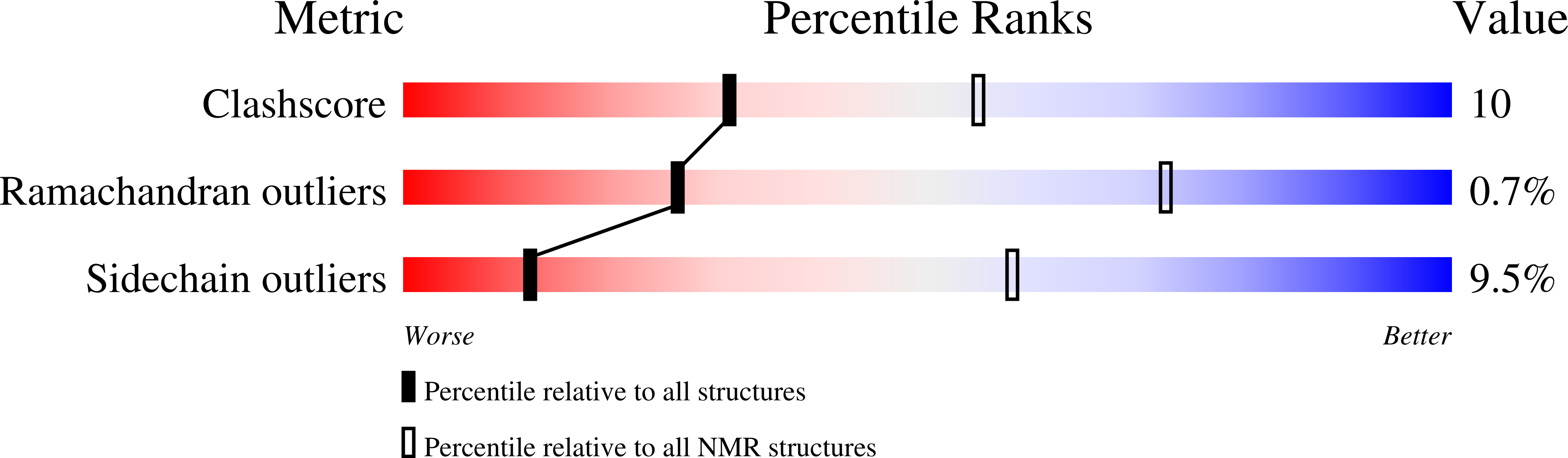Timeless couples G-quadruplex detection with processing by DDX11 helicase during DNA replication.
Lerner, L.K., Holzer, S., Kilkenny, M.L., Svikovic, S., Murat, P., Schiavone, D., Eldridge, C.B., Bittleston, A., Maman, J.D., Branzei, D., Stott, K., Pellegrini, L., Sale, J.E.(2020) EMBO J 39: e104185-e104185
- PubMed: 32705708
- DOI: https://doi.org/10.15252/embj.2019104185
- Primary Citation of Related Structures:
6T9Q, 6TAZ - PubMed Abstract:
Regions of the genome with the potential to form secondary DNA structures pose a frequent and significant impediment to DNA replication and must be actively managed in order to preserve genetic and epigenetic integrity. How the replisome detects and responds to secondary structures is poorly understood. Here, we show that a core component of the fork protection complex in the eukaryotic replisome, Timeless, harbours in its C-terminal region a previously unappreciated DNA-binding domain that exhibits specific binding to G-quadruplex (G4) DNA structures. We show that this domain contributes to maintaining processive replication through G4-forming sequences, and exhibits partial redundancy with an adjacent PARP-binding domain. Further, this function of Timeless requires interaction with and activity of the helicase DDX11. Loss of both Timeless and DDX11 causes epigenetic instability at G4-forming sequences and DNA damage. Our findings indicate that Timeless contributes to the ability of the replisome to sense replication-hindering G4 formation and ensures the prompt resolution of these structures by DDX11 to maintain processive DNA synthesis.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, UK.