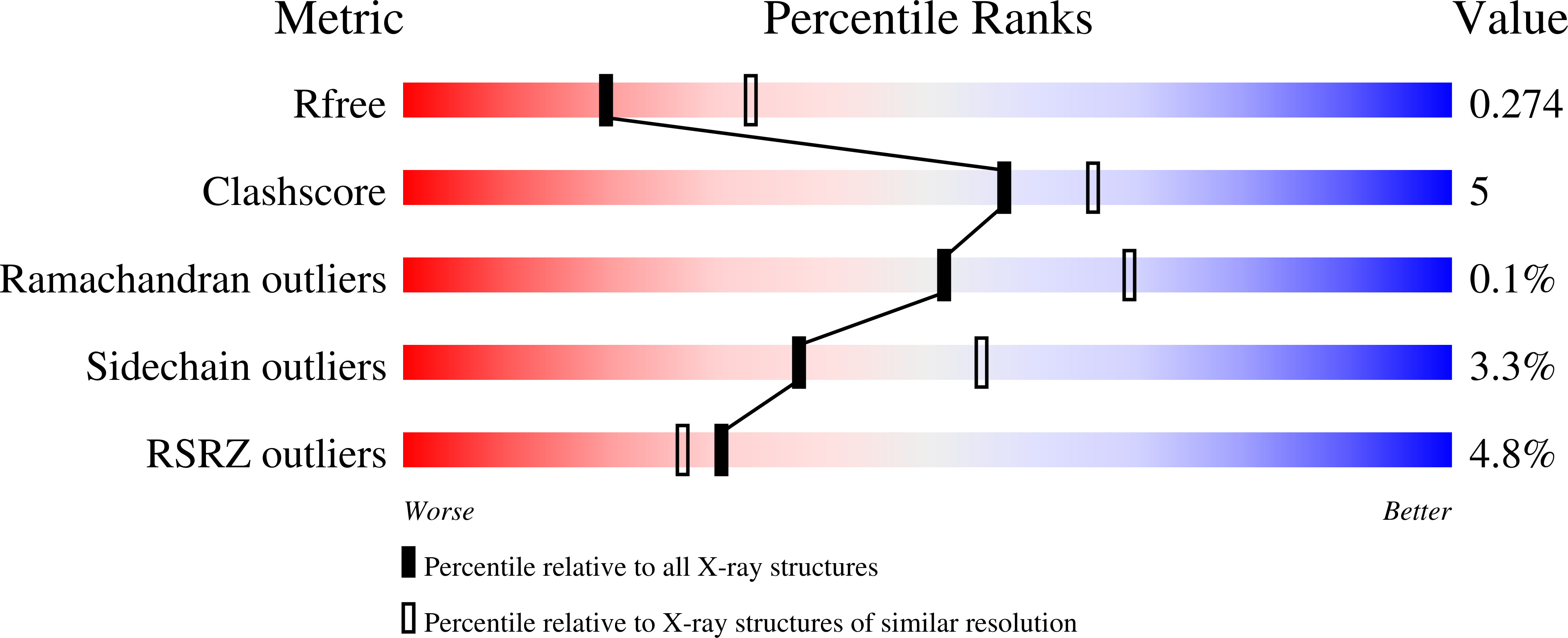
Molecular basis for the recognition of steroidogenic acute regulatory protein by the 14-3-3 protein family.
Tugaeva, K.V., Titterington, J., Sotnikov, D.V., Maksimov, E.G., Antson, A.A., Sluchanko, N.N.(2020) FEBS J 287: 3944-3966
- PubMed: 32633081
- DOI: https://doi.org/10.1111/febs.15474
- Primary Citation of Related Structures:
6T5F, 6T5H - PubMed Abstract:
Steroidogenesis in adrenals and gonads starts from cholesterol transport to mitochondria. This is mediated by the steroidogenic acute regulatory protein (STARD1), containing a mitochondrial import sequence followed by a cholesterol-binding START domain. Although mutations in this protein have been linked to lipoid congenital adrenal hyperplasia (LCAH), the mechanism of steroidogenesis regulation by STARD1 remains debatable. It has been hypothesized to involve a molten-globule structural transition and interaction with 14-3-3 proteins. In this study, we aimed to address the structural basis for the 14-3-3-STARD1 interaction. We show that, while the isolated START domain does not interact with 14-3-3, this interaction is enabled by STARD1 phosphorylation at Ser57, close to the mitochondrial peptide cleavage site. Biochemical analysis of the STARD1 affinity toward 14-3-3 and crystal structures of 14-3-3 complexes with Ser57 and Ser195 phosphopeptides suggest distinct roles of site-specific phosphorylations in recruiting 14-3-3, to modulate STARD1 activity, processing and import to the mitochondria. Phosphorylation at Ser195 creates a unique conditional site that could only bind to 14-3-3 upon partial unfolding of the START domain. Overall, our findings on the interaction between 14-3-3 and STARD1 may have potential clinical implications for patients with LCAH.
Organizational Affiliation:
Federal Research Center of Biotechnology of the Russian Academy of Sciences, A.N. Bach Institute of Biochemistry, Moscow, Russia.