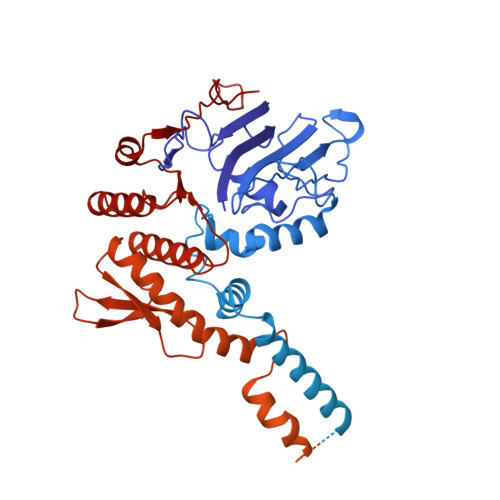
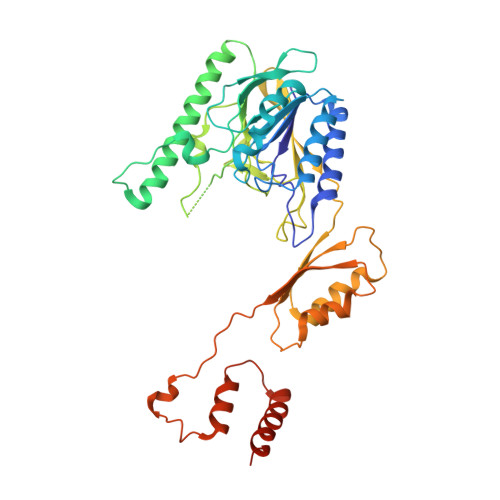
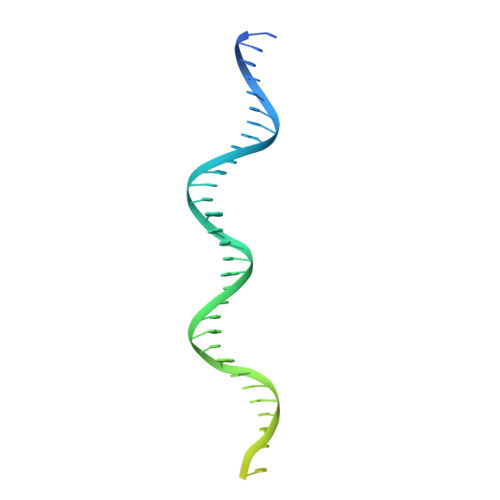
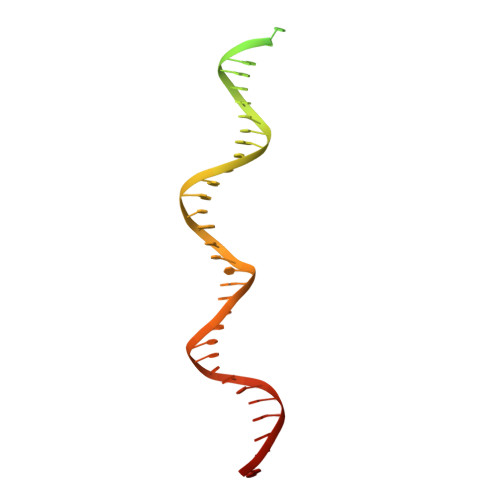
Mechanism of DNA End Sensing and Processing by the Mre11-Rad50 Complex.
Kashammer, L., Saathoff, J.H., Lammens, K., Gut, F., Bartho, J., Alt, A., Kessler, B., Hopfner, K.P.(2019) Mol Cell 76: 382
- PubMed: 31492634
- DOI: https://doi.org/10.1016/j.molcel.2019.07.035
- Primary Citation of Related Structures:
6S6V, 6S85 - PubMed Abstract:
DNA double-strand breaks (DSBs) threaten genome stability throughout life and are linked to tumorigenesis in humans. To initiate DSB repair by end joining or homologous recombination, the Mre11-nuclease Rad50-ATPase complex detects and processes diverse and obstructed DNA ends, but a structural mechanism is still lacking. Here we report cryo-EM structures of the E. coli Mre11-Rad50 homolog SbcCD in resting and DNA-bound cutting states. In the resting state, Mre11's nuclease is blocked by ATP-Rad50, and the Rad50 coiled coils appear flexible. Upon DNA binding, the two coiled coils zip up into a rod and, together with the Rad50 nucleotide-binding domains, form a clamp around dsDNA. Mre11 moves to the side of Rad50, binds the DNA end, and assembles a DNA cutting channel for the nuclease reactions. The structures reveal how Mre11-Rad50 can detect and process diverse DNA ends and uncover a clamping and gating function for the coiled coils.
Organizational Affiliation:
Department of Biochemistry, Ludwig-Maximilians-Universität, 81377 Munich, Germany; Gene Center, Ludwig-Maximilians-Universität, 81377 Munich, Germany.