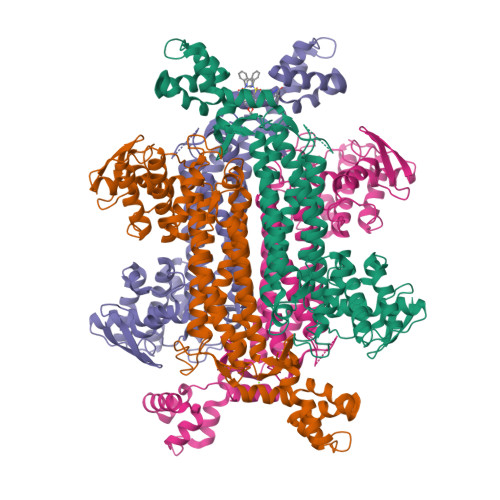
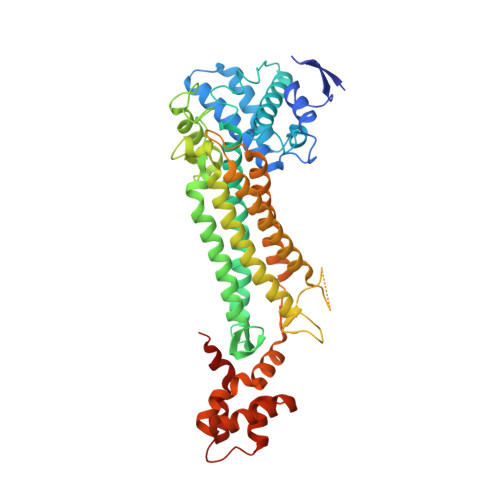
Targeting of Fumarate Hydratase fromMycobacterium tuberculosisUsing Allosteric Inhibitors with a Dimeric-Binding Mode.
Whitehouse, A.J., Libardo, M.D.J., Kasbekar, M., Brear, P.D., Fischer, G., Thomas, C.J., Barry 3rd, C.E., Boshoff, H.I.M., Coyne, A.G., Abell, C.(2019) J Med Chem 62: 10586-10604
- PubMed: 31517489
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01203
- Primary Citation of Related Structures:
6S43, 6S7K, 6S7S, 6S7U, 6S7W, 6S7Z, 6S88 - PubMed Abstract:
With the growing worldwide prevalence of antibiotic-resistant strains of tuberculosis (TB), new targets are urgently required for the development of treatments with novel modes of action. Fumarate hydratase (fumarase), a vulnerable component of the citric acid cycle in Mycobacterium tuberculosis ( Mtb ), is a metabolic target that could satisfy this unmet demand. A key challenge in the targeting of Mtb fumarase is its similarity to the human homolog, which shares an identical active site. A potential solution to this selectivity problem was previously found in a high-throughput screening hit that binds in a nonconserved allosteric site. In this work, a structure-activity relationship study was carried out with the determination of further structural biology on the lead series, affording derivatives with sub-micromolar inhibition. Further, the screening of this series against Mtb in vitro identified compounds with potent minimum inhibitory concentrations.
Organizational Affiliation:
Department of Chemistry , University of Cambridge , Lensfield Road , Cambridge CB2 1EW , U.K.