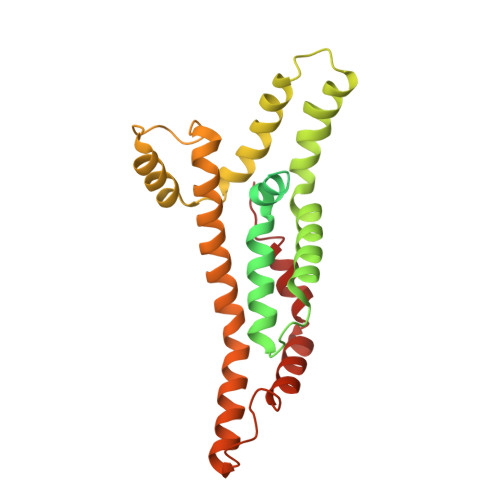
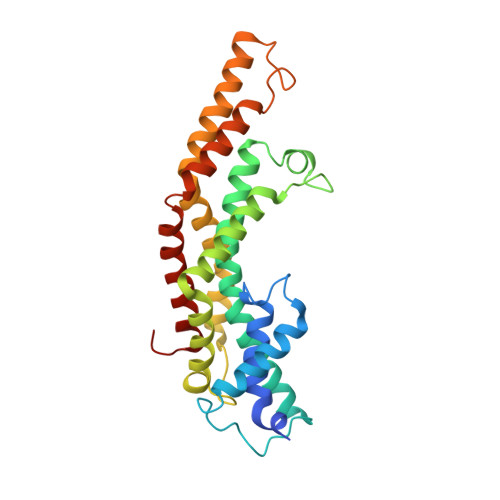
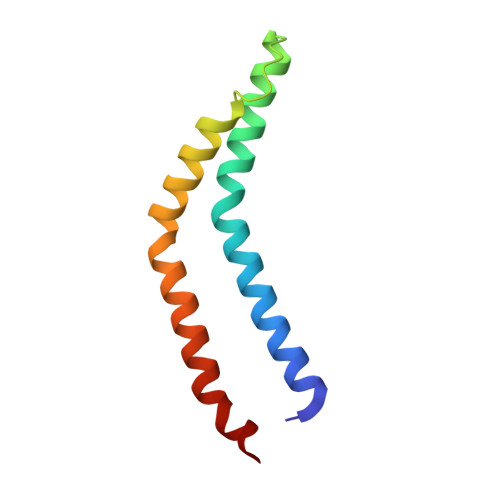
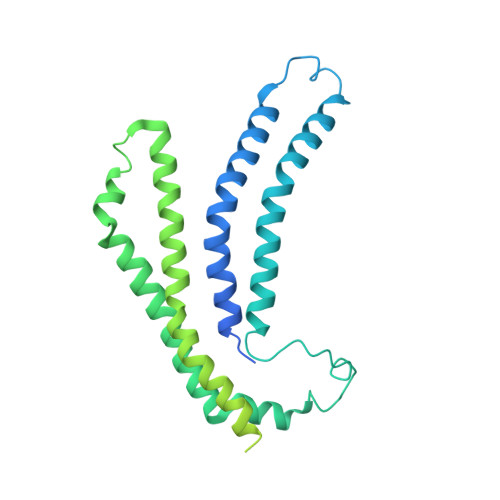
The substrate specificity switch FlhB assembles onto the export gate to regulate type three secretion.
Kuhlen, L., Johnson, S., Zeitler, A., Baurle, S., Deme, J.C., Caesar, J.J.E., Debo, R., Fisher, J., Wagner, S., Lea, S.M.(2020) Nat Commun 11: 1296-1296
- PubMed: 32157081
- DOI: https://doi.org/10.1038/s41467-020-15071-9
- Primary Citation of Related Structures:
6S3L, 6S3R, 6S3S - PubMed Abstract:
Protein secretion through type-three secretion systems (T3SS) is critical for motility and virulence of many bacteria. Proteins are transported through an export gate containing three proteins (FliPQR in flagella, SctRST in virulence systems). A fourth essential T3SS protein (FlhB/SctU) functions to "switch" secretion substrate specificity once the growing hook/needle reach their determined length. Here, we present the cryo-electron microscopy structure of an export gate containing the switch protein from a Vibrio flagellar system at 3.2 Å resolution. The structure reveals that FlhB/SctU extends the helical export gate with its four predicted transmembrane helices wrapped around FliPQR/SctRST. The unusual topology of the FlhB/SctU helices creates a loop wrapped around the bottom of the closed export gate. Structure-informed mutagenesis suggests that this loop is critical in gating secretion and we propose that a series of conformational changes in the T3SS trigger opening of the gate through interactions between FlhB/SctU and FliPQR/SctRST.
Organizational Affiliation:
Sir William Dunn School of Pathology, University of Oxford, Oxford, OX13RE, UK.