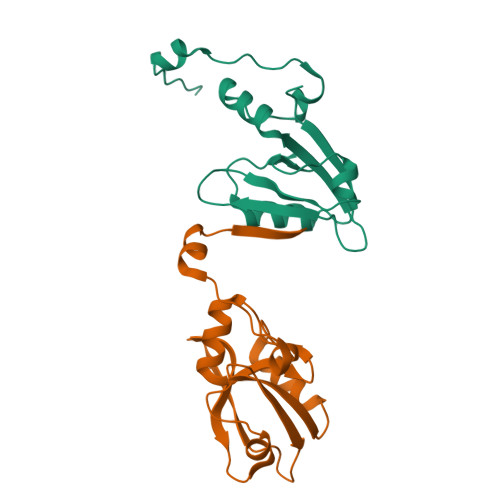
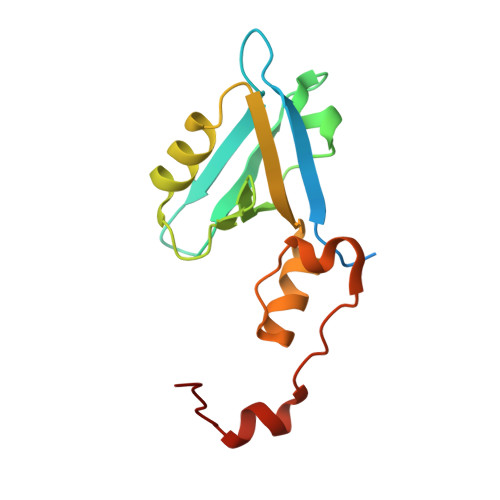
In vivocrystals reveal critical features of the interaction between cystic fibrosis transmembrane conductance regulator (CFTR) and the PDZ2 domain of Na+/H+exchange cofactor NHERF1.
Martin, E.R., Barbieri, A., Ford, R.C., Robinson, R.C.(2020) J Biol Chem 295: 4464-4476
- PubMed: 32014995
- DOI: https://doi.org/10.1074/jbc.RA119.012015
- Primary Citation of Related Structures:
6RQR - PubMed Abstract:
Crystallization of recombinant proteins has been fundamental to our understanding of protein function, dysfunction, and molecular recognition. However, this information has often been gleaned under extremely nonphysiological protein, salt, and H + concentrations. Here, we describe the development of a robust Inka1-Box (iBox)-PAK4cat system that spontaneously crystallizes in several mammalian cell types. The semi-quantitative assay described here allows the measurement of in vivo protein-protein interactions using a novel GFP-linked reporter system that produces fluorescent readouts from protein crystals. We combined this assay with in vitro X-ray crystallography and molecular dynamics studies to characterize the molecular determinants of the interaction between the PDZ2 domain of Na + /H + exchange regulatory cofactor NHE-RF1 (NHERF1) and cystic fibrosis transmembrane conductance regulator (CFTR), a protein complex pertinent to the genetic disease cystic fibrosis. These experiments revealed the crystal structure of the extended PDZ domain of NHERF1 and indicated, contrary to what has been previously reported, that residue selection at positions -1 and -3 of the PDZ-binding motif influences the affinity and specificity of the NHERF1 PDZ2-CFTR interaction. Our results suggest that this system could be utilized to screen additional protein-protein interactions, provided they can be accommodated within the spacious iBox-PAK4cat lattice.
Organizational Affiliation:
School of Biological Sciences, Faculty of Biology Medicine and Health, Michael Smith Building, The University of Manchester, Manchester, M13 9PL, United Kingdom.