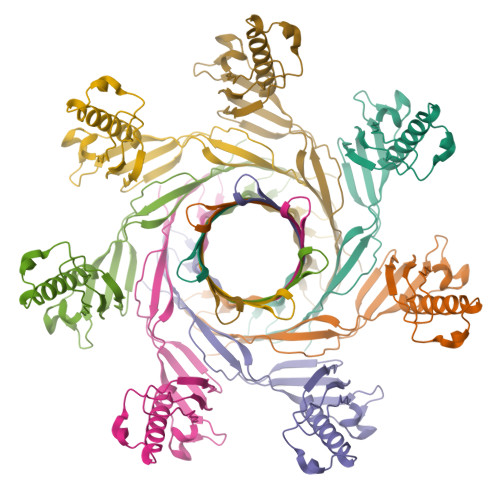
The pore structure of Clostridium perfringens epsilon toxin.
Savva, C.G., Clark, A.R., Naylor, C.E., Popoff, M.R., Moss, D.S., Basak, A.K., Titball, R.W., Bokori-Brown, M.(2019) Nat Commun 10: 2641-2641
- PubMed: 31201325
- DOI: https://doi.org/10.1038/s41467-019-10645-8
- Primary Citation of Related Structures:
6RB9 - PubMed Abstract:
Epsilon toxin (Etx), a potent pore forming toxin (PFT) produced by Clostridium perfringens, is responsible for the pathogenesis of enterotoxaemia of ruminants and has been suggested to play a role in multiple sclerosis in humans. Etx is a member of the aerolysin family of β-PFTs (aβ-PFTs). While the Etx soluble monomer structure was solved in 2004, Etx pore structure has remained elusive due to the difficulty of isolating the pore complex. Here we show the cryo-electron microscopy structure of Etx pore assembled on the membrane of susceptible cells. The pore structure explains important mutant phenotypes and suggests that the double β-barrel, a common feature of the aβ-PFTs, may be an important structural element in driving efficient pore formation. These insights provide the framework for the development of novel therapeutics to prevent human and animal infections, and are relevant for nano-biotechnology applications.
Organizational Affiliation:
Leicester Institute of Structural and Chemical Biology, Department of Molecular and Cell Biology, University of Leicester, Lancaster Road, Leicester, LE1 7HB, UK.