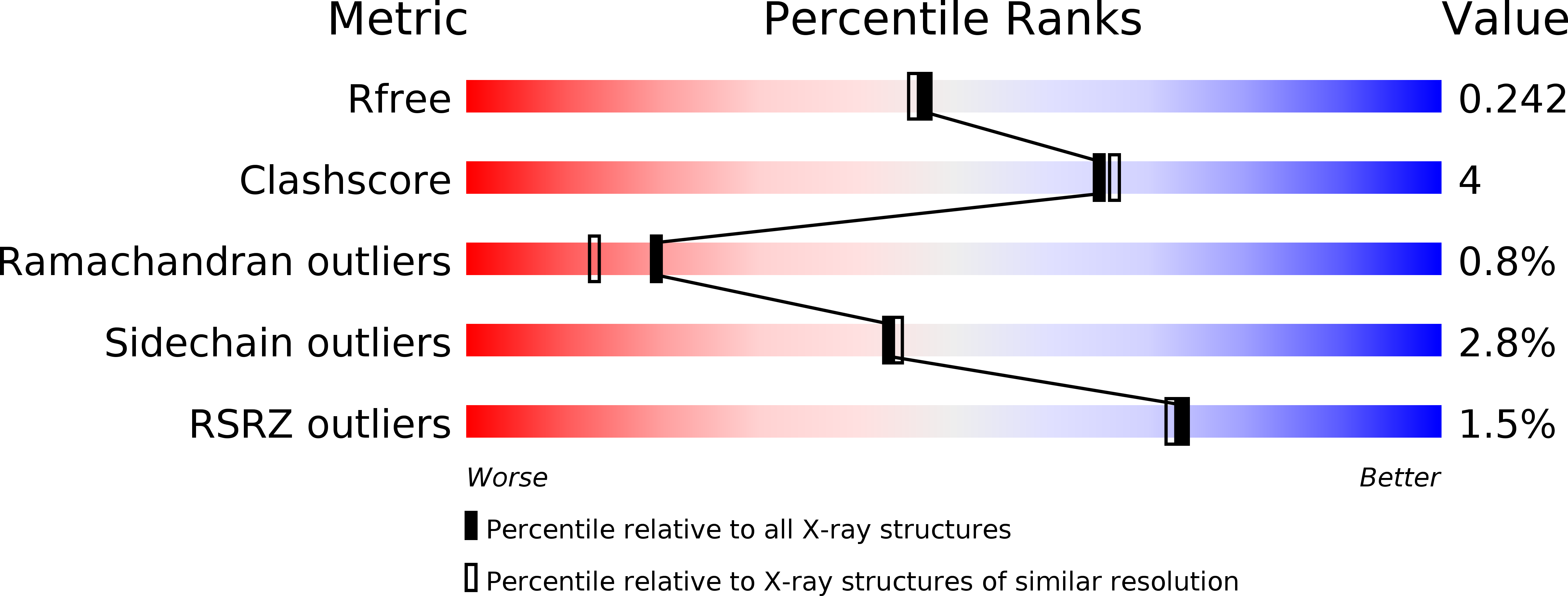
Structural basis for recognition and ring-cleavage of the Pseudomonas quinolone signal (PQS) by AqdC, a mycobacterial dioxygenase of the alpha / beta-hydrolase fold family.
Wullich, S.C., Kobus, S., Wienhold, M., Hennecke, U., Smits, S.H.J., Fetzner, S.(2019) J Struct Biol 207: 287-294
- PubMed: 31228546
- DOI: https://doi.org/10.1016/j.jsb.2019.06.006
- Primary Citation of Related Structures:
6RA2, 6RA3, 6RB3 - PubMed Abstract:
The cofactor-less dioxygenase AqdC of Mycobacteroides abscessus catalyzes the cleavage and thus inactivation of the Pseudomonas quinolone signal (PQS, 2-heptyl-3-hydroxy-4(1H)-quinolone), which plays a central role in the regulation of virulence factor production by Pseudomonas aeruginosa. We present here the crystal structures of AqdC in its native state and in complex with the PQS cleavage product N-octanoylanthranilic acid, and of mutant AqdC proteins in complex with PQS. AqdC possesses an α/β-hydrolase fold core domain with additional helices forming a cap domain. The protein is traversed by a bipartite tunnel, with a funnel-like entry section leading to an elliptical substrate cavity where PQS positioning is mediated by a combination of hydrophobic interactions and hydrogen bonds, with the substrate's C4 carbonyl and C3 hydroxyl groups tethered by His97 and the catalytic His246, respectively. The side chain of the AqdC-bound product extends deeper into the "alkyl tail section" of the tunnel than PQS, tentatively suggesting product exit via this part of the tunnel. AqdC prefers PQS over congeners with shorter alkyl substituents at C2. Kinetic data confirmed the strict requirement of the active-site base His246 for catalysis, and suggested that evolution of the canonical nucleophile/His/Asp catalytic triad of the hydrolases to an Ala/His/Asp triad is favorable for catalyzing dioxygenolytic PQS ring cleavage.
Organizational Affiliation:
Institute for Molecular Microbiology and Biotechnology, University of Münster, Corrensstraße 3, 48149 Münster, Germany. Electronic address: wullich@wwu.de.