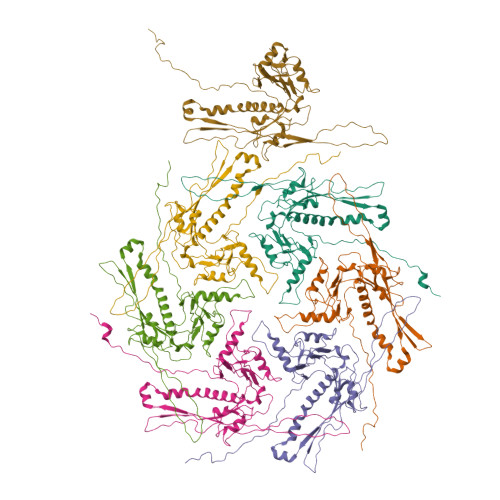
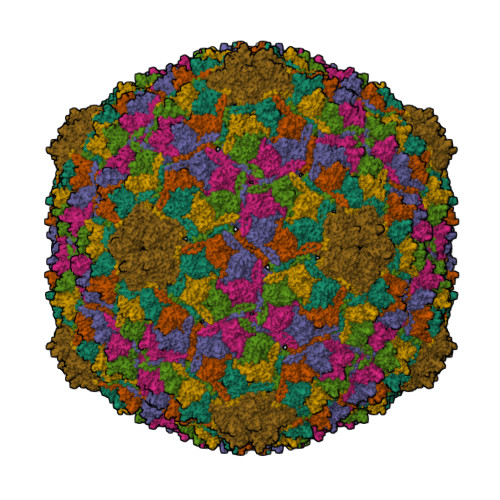
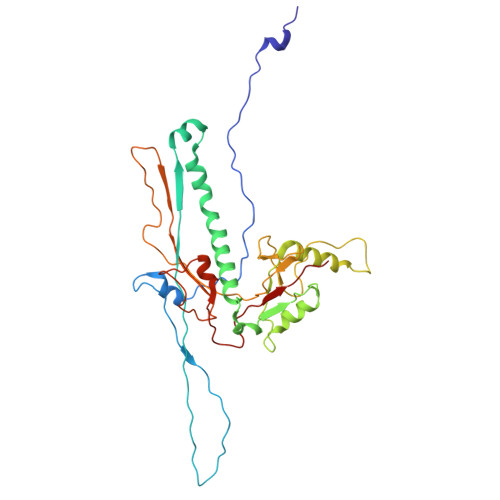
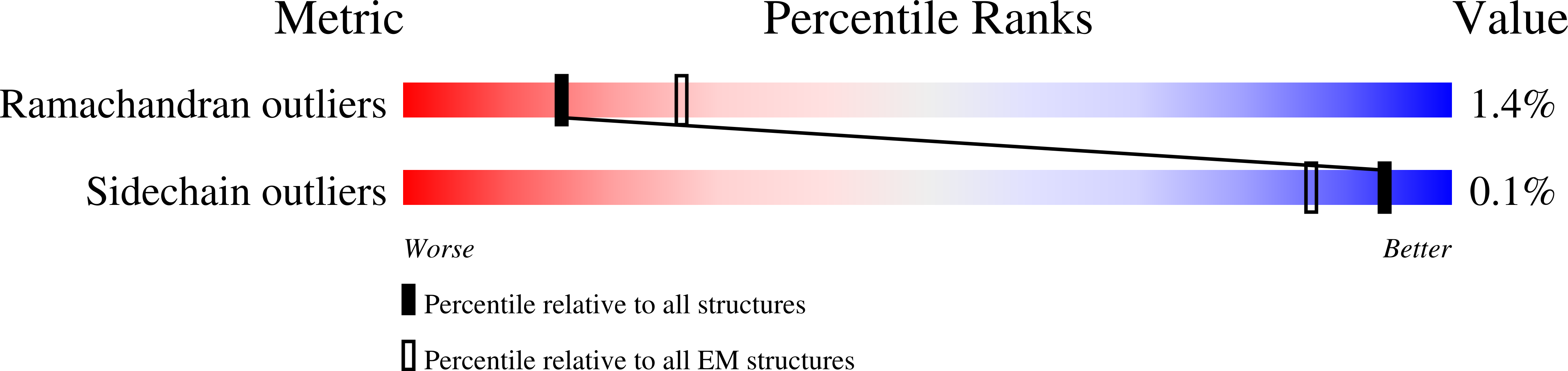Structural transitions during the scaffolding-driven assembly of a viral capsid.
Ignatiou, A., Brasiles, S., El Sadek Fadel, M., Burger, J., Mielke, T., Topf, M., Tavares, P., Orlova, E.V.(2019) Nat Commun 10: 4840-4840
- PubMed: 31649265
- DOI: https://doi.org/10.1038/s41467-019-12790-6
- Primary Citation of Related Structures:
6R3A, 6R3B, 6RTL - PubMed Abstract:
Assembly of tailed bacteriophages and herpesviruses starts with formation of procapsids (virion precursors without DNA). Scaffolding proteins (SP) drive assembly by chaperoning the major capsid protein (MCP) to build an icosahedral lattice. Here we report near-atomic resolution cryo-EM structures of the bacteriophage SPP1 procapsid, the intermediate expanded procapsid with partially released SPs, and the mature capsid with DNA. In the intermediate state, SPs are bound only to MCP pentons and to adjacent subunits from hexons. SP departure results in the expanded state associated with unfolding of the MCP N-terminus and straightening of E-loops. The newly formed extensive inter-capsomere bonding appears to compensate for release of SPs that clasp MCP capsomeres together. Subsequent DNA packaging instigates bending of MCP A domain loops outwards, closing the hexons central opening and creating the capsid auxiliary protein binding interface. These findings provide a molecular basis for the sequential structural rearrangements during viral capsid maturation.
Organizational Affiliation:
Institute of Structural and Molecular Biology, Birkbeck College, Malet Street, London, WC1E 7HX, UK.