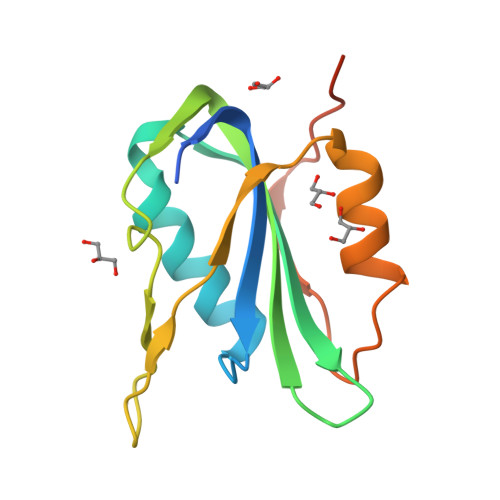
Crystal structure of the Wheat dwarf virus Rep domain.
Everett, B.A., Litzau, L.A., Tompkins, K., Shi, K., Nelson, A., Aihara, H., Evans Iii, R.L., Gordon, W.R.(2019) Acta Crystallogr F Struct Biol Commun 75: 744-749
- PubMed: 31797816
- DOI: https://doi.org/10.1107/S2053230X19015796
- Primary Citation of Related Structures:
6Q1M - PubMed Abstract:
The Rep domain of Wheat dwarf virus (WDV Rep) is an HUH endonuclease involved in rolling-circle replication. HUH endonucleases coordinate a metal ion to enable the nicking of a specific ssDNA sequence and the subsequent formation of an intermediate phosphotyrosine bond. This covalent protein-ssDNA adduct makes HUH endonucleases attractive fusion tags (HUH-tags) in a diverse number of biotechnological applications. Solving the structure of an HUH endonuclease in complex with ssDNA will provide critical information about ssDNA recognition and sequence specificity, thus enabling rationally engineered protein-DNA interactions that are programmable. The structure of the WDV Rep domain reported here was solved in the apo state from a crystal diffracting to 1.24 Å resolution and represents an initial step in the direction of solving the structure of a protein-ssDNA complex.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, Minnesota, USA.