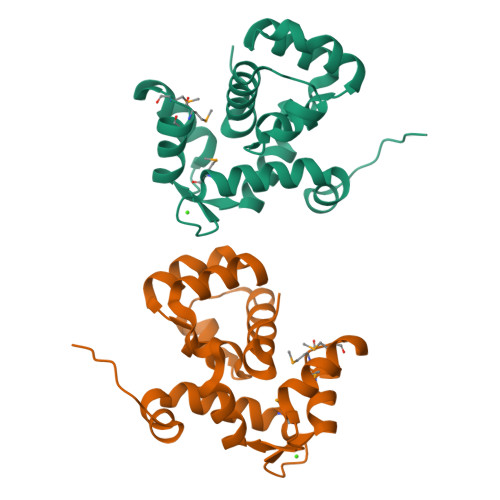
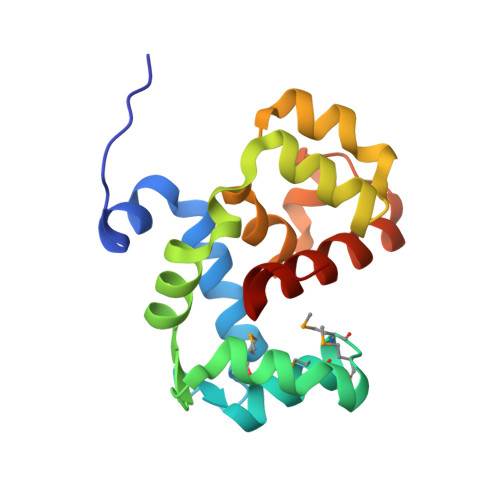
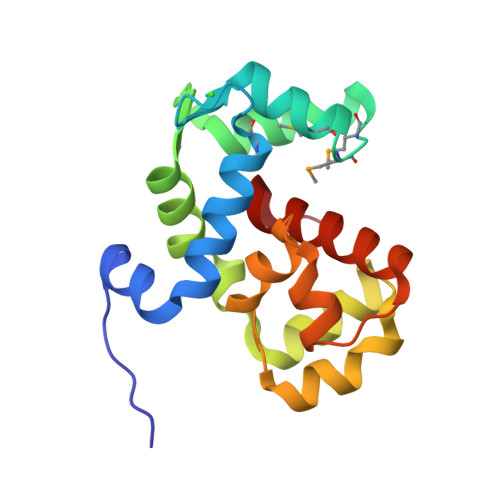
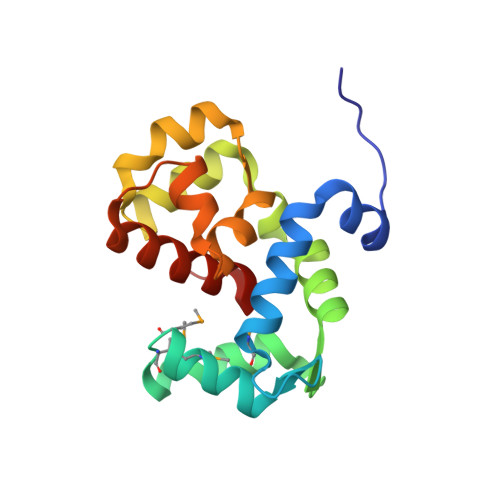
Coordination of a Single Calcium Ion in the EF-hand Maintains the Off State of the Stromal Interaction Molecule Luminal Domain.
Enomoto, M., Nishikawa, T., Back, S.I., Ishiyama, N., Zheng, L., Stathopulos, P.B., Ikura, M.(2020) J Mol Biology 432: 367-383
- PubMed: 31626806
- DOI: https://doi.org/10.1016/j.jmb.2019.10.003
- Primary Citation of Related Structures:
6PW7 - PubMed Abstract:
Store operated calcium (Ca 2+ ) entry (SOCE) is the process whereby endoplasmic reticulum (ER) Ca 2+ store depletion causes Orai1-composed Ca 2+ channels on the plasma membrane (PM) to open, mediating a rise in cytosolic Ca 2+ levels. Stromal interaction molecules (STIMs) are the proteins that directly sense ER Ca 2+ content and gate Orai1 channels due to store depletion. The trigger for STIM activation is Ca 2+ unbinding from the ER lumen-oriented domains, which consist of a nonconserved amino (N) terminal region and EF-hand and sterile α motif (SAM) domains (EF-SAM), highly conserved from humans to Caenorhabditis elegans. Solution NMR structures of the human EF-SAM domains have been determined at high Ca 2+ concentrations; however, no direct structural view of the Ca 2+ binding mode has been elucidated. Further, no atomic resolution data currently exists on EF-SAM at low Ca 2+ levels. Here, we determined the X-ray crystal structure of the C. elegans STIM luminal domain, revealing that EF-SAM binds a single Ca 2+ ion with pentagonal bipyramidal geometry and an ancillary α-helix formed by the N-terminal region acts as a brace to stabilize EF-SAM. Using solution NMR, we observed EF-hand domain unfolding and a conformational exchange between folded and unfolded states involving the ancillary α-helix and the canonical EF-hand in low Ca 2+ . Remarkably, we also detected an α-helix (+Ca 2+ ) to β-strand (-Ca 2+ ) transition at the terminal SAM domain α-helix. Collectively, our analyses indicate that one canonically bound Ca 2+ ion is sufficient to stabilize the quiescent luminal domain structure, precluding unfolding, conformational exchange, and secondary structure transformation.
Organizational Affiliation:
Princess Margaret Cancer Centre, Department of Medical Biophysics, University Health Network, University of Toronto, Toronto, ON M5G 1L7, Canada.