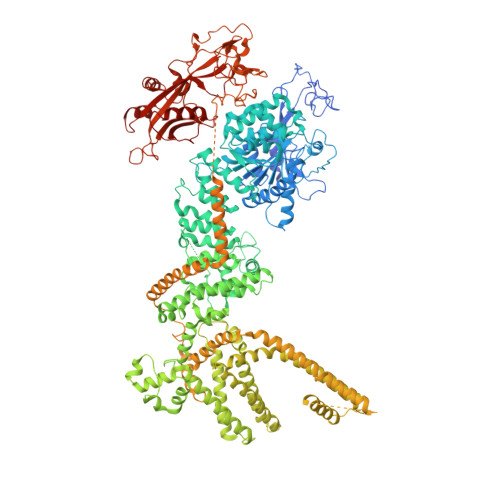
Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel.
Huang, Y., Roth, B., Lu, W., Du, J.(2019) Elife 8
- PubMed: 31513012
- DOI: https://doi.org/10.7554/eLife.50175
- Primary Citation of Related Structures:
6PUO, 6PUR, 6PUS, 6PUU - PubMed Abstract:
TRPM2 is critically involved in diverse physiological processes including core temperature sensing, apoptosis, and immune response. TRPM2's activation by Ca 2+ and ADP ribose (ADPR), an NAD + -metabolite produced under oxidative stress and neurodegenerative conditions, suggests a role in neurological disorders. We provide a central concept between triple-site ligand binding and the channel gating of human TRPM2. We show consecutive structural rearrangements and channel activation of TRPM2 induced by binding of ADPR in two indispensable locations, and the binding of Ca 2+ in the transmembrane domain. The 8-Br-cADPR-an antagonist of cADPR-binds only to the MHR1/2 domain and inhibits TRPM2 by stabilizing the channel in an apo-like conformation. We conclude that MHR1/2 acts as a orthostatic ligand-binding site for TRPM2. The NUDT9-H domain binds to a second ADPR to assist channel activation in vertebrates, but not necessary in invertebrates. Our work provides insights into the gating mechanism of human TRPM2 and its pharmacology.
Organizational Affiliation:
Van Andel Institute, Grand Rapids, United States.