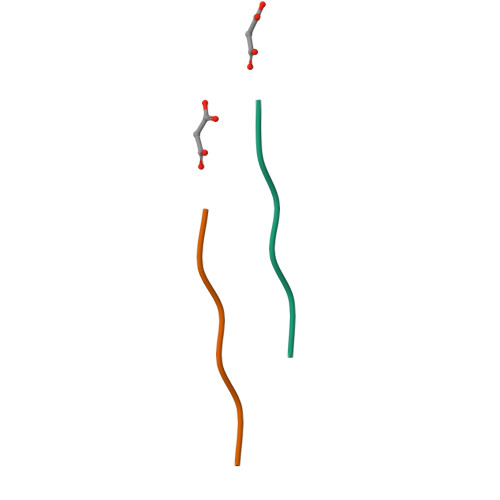
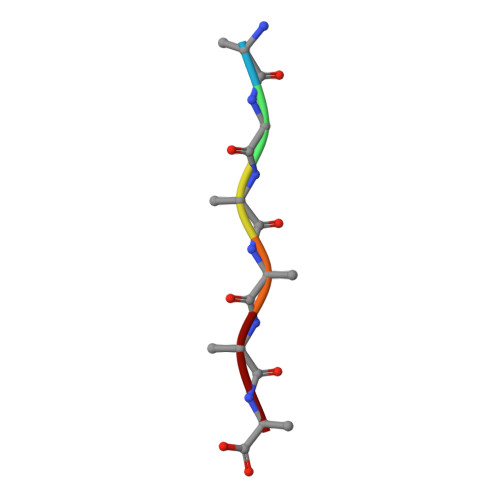
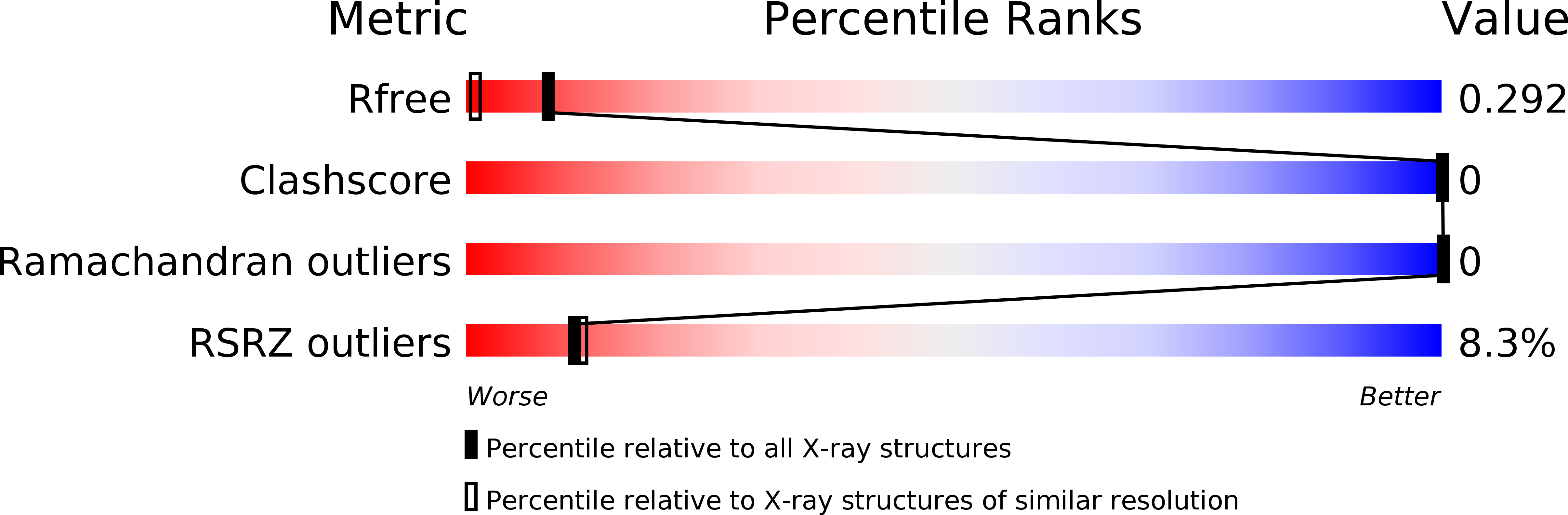Cryo-EM structure of a human prion fibril with a hydrophobic, protease-resistant core.
Glynn, C., Sawaya, M.R., Ge, P., Gallagher-Jones, M., Short, C.W., Bowman, R., Apostol, M., Zhou, Z.H., Eisenberg, D.S., Rodriguez, J.A.(2020) Nat Struct Mol Biol 27: 417-423
- PubMed: 32284600
- DOI: https://doi.org/10.1038/s41594-020-0403-y
- Primary Citation of Related Structures:
6PQ5, 6PQA, 6UUR - PubMed Abstract:
Self-templating assemblies of the human prion protein are clinically associated with transmissible spongiform encephalopathies. Here we present the cryo-EM structure of a denaturant- and protease-resistant fibril formed in vitro spontaneously by a 9.7-kDa unglycosylated fragment of the human prion protein. This human prion fibril contains two protofilaments intertwined with screw symmetry and linked by a tightly packed hydrophobic interface. Each protofilament consists of an extended beta arch formed by residues 106 to 145 of the prion protein, a hydrophobic and highly fibrillogenic disease-associated segment. Such structures of prion polymorphs serve as blueprints on which to evaluate the potential impact of sequence variants on prion disease.
Organizational Affiliation:
Department of Chemistry and Biochemistry; UCLA-DOE Institute for Genomics and Proteomics; STROBE, NSF Science and Technology Center, University of California, Los Angeles, Los Angeles, CA, USA.