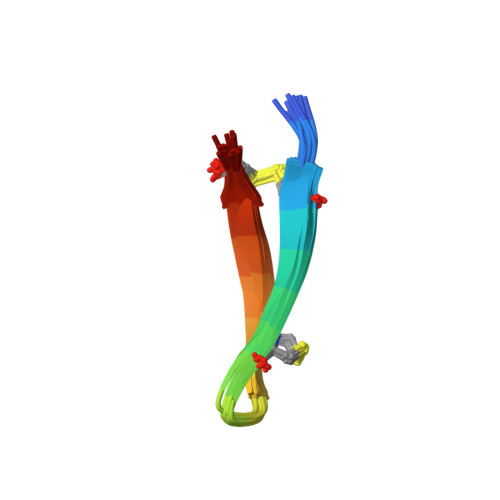
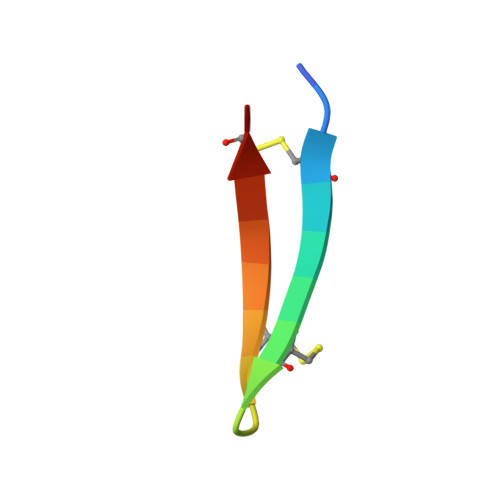
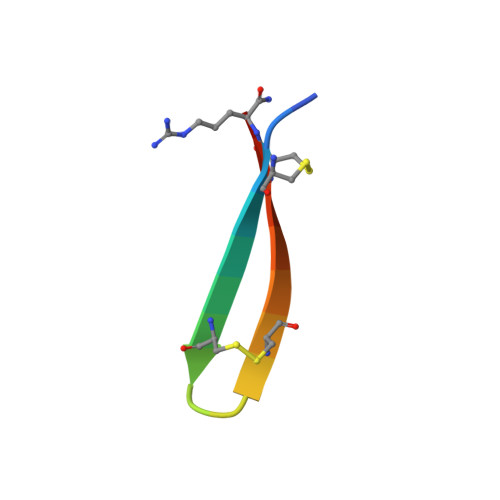
Characterization of Tachyplesin Peptides and Their Cyclized Analogues to Improve Antimicrobial and Anticancer Properties.
Vernen, F., Harvey, P.J., Dias, S.A., Veiga, A.S., Huang, Y.H., Craik, D.J., Lawrence, N., Troeira Henriques, S.(2019) Int J Mol Sci 20
- PubMed: 31455019
- DOI: https://doi.org/10.3390/ijms20174184
- Primary Citation of Related Structures:
6PI2, 6PI3, 6PIN, 6PIO, 6PIP - PubMed Abstract:
Tachyplesin I, II and III are host defense peptides from horseshoe crab species with antimicrobial and anticancer activities. They have an amphipathic β-hairpin structure, are highly positively-charged and differ by only one or two amino acid residues. In this study, we compared the structure and activity of the three tachyplesin peptides alongside their backbone cyclized analogues. We assessed the peptide structures using nuclear magnetic resonance (NMR) spectroscopy, then compared the activity against bacteria (both in the planktonic and biofilm forms) and a panel of cancerous cells. The importance of peptide-lipid interactions was examined using surface plasmon resonance and fluorescence spectroscopy methodologies. Our studies showed that tachyplesin peptides and their cyclic analogues were most potent against Gram-negative bacteria and melanoma cell lines, and showed a preference for binding to negatively-charged lipid membranes. Backbone cyclization did not improve potency, but improved peptide stability in human serum and reduced toxicity toward human red blood cells. Peptide-lipid binding affinity, orientation within the membrane, and ability to disrupt lipid bilayers differed between the cyclized peptide and the parent counterpart. We show that tachyplesin peptides and cyclized analogues have similarly potent antimicrobial and anticancer properties, but that backbone cyclization improves their stability and therapeutic potential.
Organizational Affiliation:
Institute for Molecular Bioscience, The University of Queensland, Brisbane, Queensland 4072, Australia.