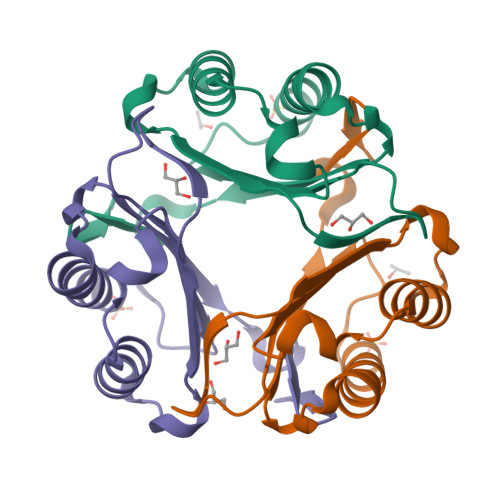
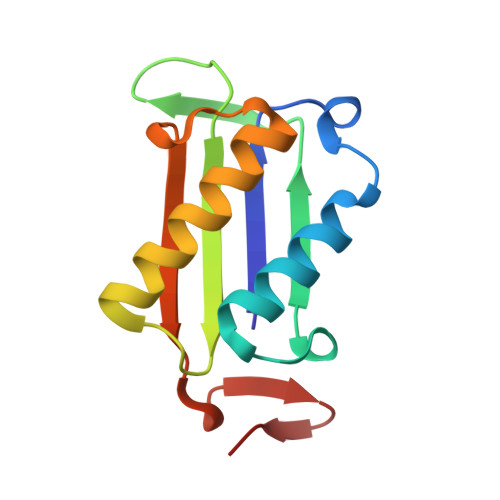
Regulation of MIF Enzymatic Activity by an Allosteric Site at the Central Solvent Channel.
Pantouris, G., Khurana, L., Ma, A., Skeens, E., Reiss, K., Batista, V.S., Lisi, G.P., Lolis, E.J.(2020) Cell Chem Biol 27: 740-750.e5
- PubMed: 32433911
- DOI: https://doi.org/10.1016/j.chembiol.2020.05.001
- Primary Citation of Related Structures:
5UMJ, 5UMK, 6OY8, 6OYB, 6OYE, 6OYG - PubMed Abstract:
In proteins with multiple functions, such as macrophage migration inhibitory factor (MIF), the study of its intramolecular dynamic network can offer a unique opportunity to understand how a single protein is able to carry out several nonoverlapping functions. A dynamic mechanism that controls the MIF-induced activation of CD74 was recently discovered. In this study, the regulation of tautomerase activity was explored. The catalytic base Pro1 is found to form dynamic communications with the same allosteric node that regulates CD74 activation. Signal transmission between the allosteric and catalytic sites take place through intramolecular aromatic interactions and a hydrogen bond network that involves residues and water molecules of the MIF solvent channel. Once thought to be a consequence of trimerization, a regulatory function for the solvent channel is now defined. These results provide mechanistic insights into the regulation of catalytic activity and the role of solvent channel water molecules in MIF catalysis.
Organizational Affiliation:
Department of Chemistry, University of the Pacific, Stockton, CA 95211, USA. Electronic address: gpantouris@pacific.edu.