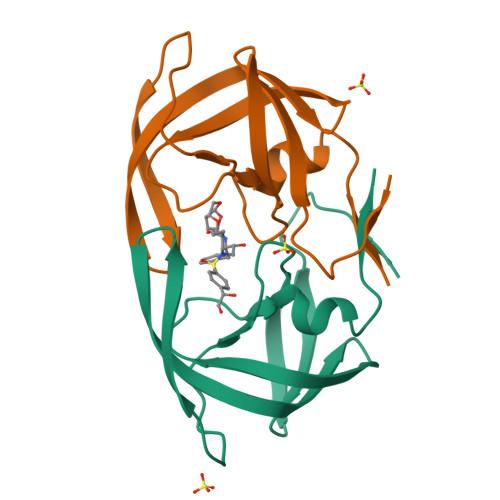
HIV-1 Protease Inhibitors Incorporating Stereochemically Defined P2' Ligands To Optimize Hydrogen Bonding in the Substrate Envelope.
Rusere, L.N., Lockbaum, G.J., Lee, S.K., Henes, M., Kosovrasti, K., Spielvogel, E., Nalivaika, E.A., Swanstrom, R., Yilmaz, N.K., Schiffer, C.A., Ali, A.(2019) J Med Chem 62: 8062-8079
- PubMed: 31386368
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00838
- Primary Citation of Related Structures:
6OXO, 6OXP, 6OXQ, 6OXR, 6OXS, 6OXT, 6OXU, 6OXV, 6OXW, 6OXX, 6OXY, 6OXZ, 6OY0, 6OY1, 6OY2 - PubMed Abstract:
A structure-guided design strategy was used to improve the resistance profile of HIV-1 protease inhibitors by optimizing hydrogen bonding and van der Waals interactions with the protease while staying within the substrate envelope. Stereoisomers of 4-(1-hydroxyethyl)benzene and 4-(1,2-dihydroxyethyl)benzene moieties were explored as P2' ligands providing pairs of diastereoisomers epimeric at P2', which exhibited distinct potency profiles depending on the configuration of the hydroxyl group and size of the P1' group. While compounds with the 4-(1-hydroxyethyl)benzene P2' moiety maintained excellent antiviral potency against a panel of multidrug-resistant HIV-1 strains, analogues with the polar 4-(1,2-dihydroxyethyl)benzene moiety were less potent, and only the ( R )-epimer incorporating a larger 2-ethylbutyl P1' group showed improved potency. Crystal structures of protease-inhibitor complexes revealed strong hydrogen bonding interactions of both ( R )- and ( S )-stereoisomers of the hydroxyethyl group with Asp30'. Notably, the ( R )-dihydroxyethyl group was involved in a unique pattern of direct hydrogen bonding interactions with the backbone amides of Asp29' and Asp30'. The SAR data and analysis of crystal structures provide insights for optimizing these promising HIV-1 protease inhibitors.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology , University of Massachusetts Medical School , Worcester , Massachusetts 01605 , United States.