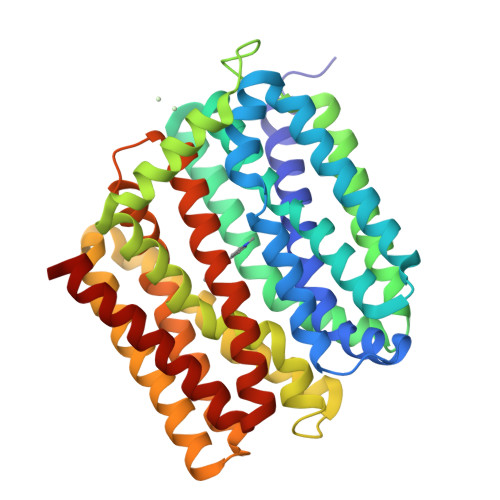
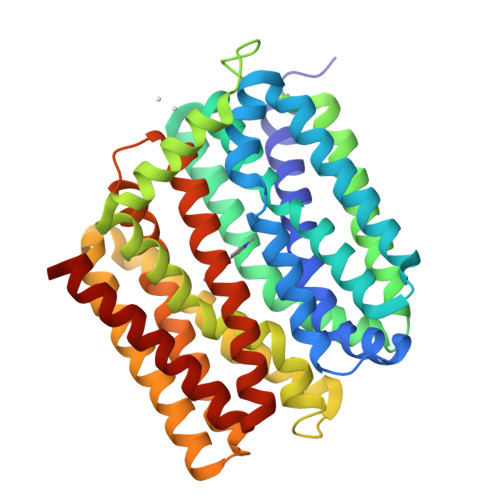
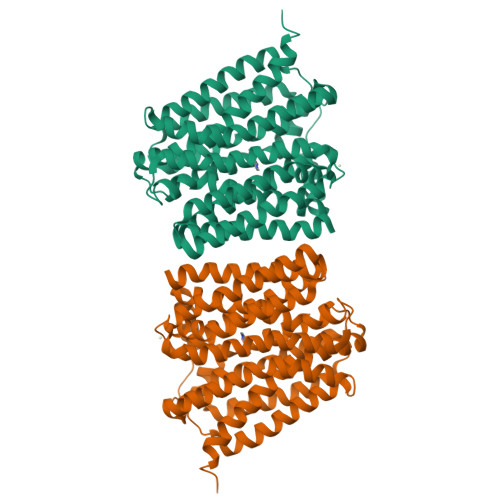
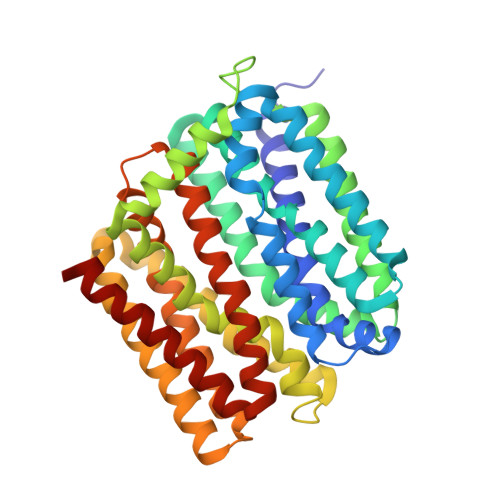
Structure of an engineered multidrug transporter MdfA reveals the molecular basis for substrate recognition.
Wu, H.H., Symersky, J., Lu, M.(2019) Commun Biol 2: 210-210
- PubMed: 31240248
- DOI: https://doi.org/10.1038/s42003-019-0446-y
- Primary Citation of Related Structures:
6OOM, 6OOP, 6OOQ - PubMed Abstract:
MdfA is a prototypical H + -coupled multidrug transporter that is characterized by extraordinarily broad substrate specificity. The involvement of specific H-bonds in MdfA-drug interactions and the simplicity of altering the substrate specificity of MdfA contradict the promiscuous nature of multidrug recognition, presenting a baffling conundrum. Here we show the X-ray structures of MdfA variant I239T/G354E in complexes with three electrically different ligands, determined at resolutions up to 2.2 Å. Our structures reveal that I239T/G354E interacts with these compounds differently from MdfA and that I239T/G354E possesses two discrete, non-overlapping substrate-binding sites. Our results shed new light on the molecular design of multidrug-binding and protonation sites and highlight the importance of often-neglected, long-range charge-charge interactions in multidrug recognition. Beyond helping to solve the ostensible conundrum of multidrug recognition, our findings suggest the mechanistic difference between substrate and inhibitor for any H + -dependent multidrug transporter, which may open new vistas on curtailing efflux-mediated multidrug resistance.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Rosalind Franklin University of Medicine and Science, 3333 Green Bay Road, North Chicago, IL 60064 USA.