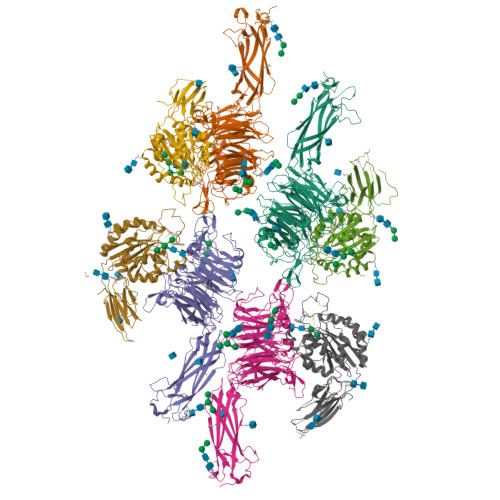
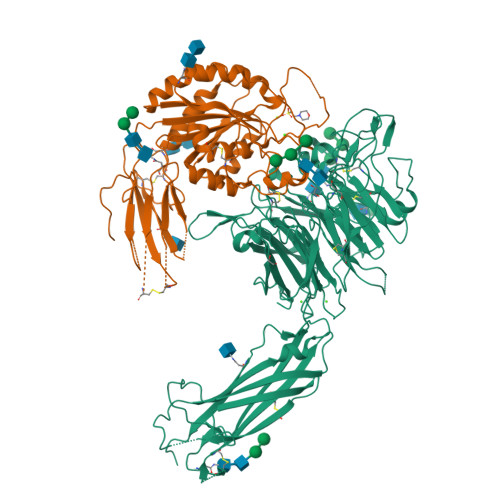
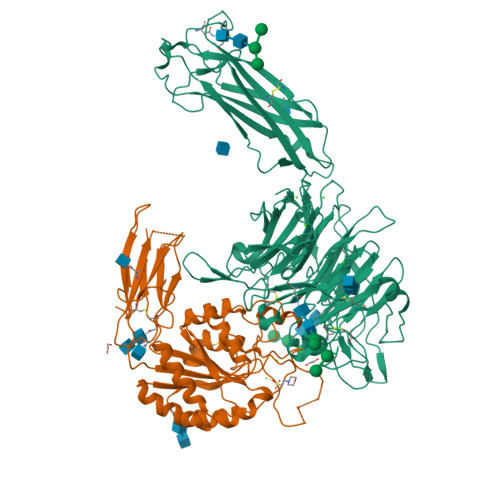
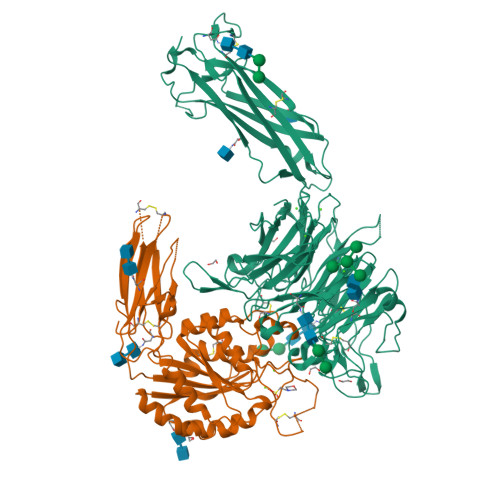
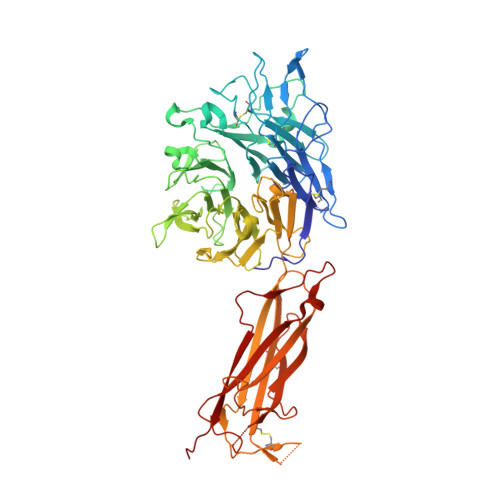
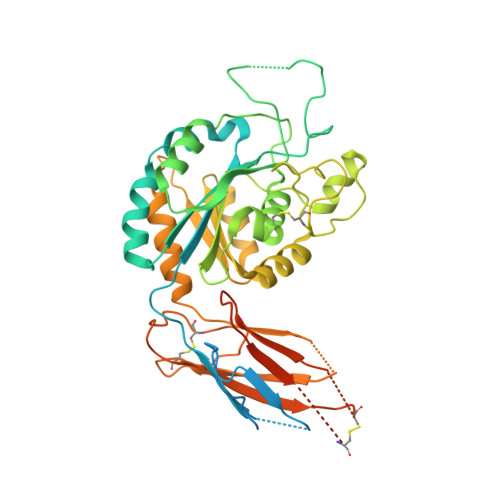
General structural features that regulate integrin affinity revealed by atypical alpha V beta 8.
Wang, J., Su, Y., Iacob, R.E., Engen, J.R., Springer, T.A.(2019) Nat Commun 10: 5481-5481
- PubMed: 31792290
- DOI: https://doi.org/10.1038/s41467-019-13248-5
- Primary Citation of Related Structures:
6OM1, 6OM2 - PubMed Abstract:
Integrin αVβ8, which like αVβ6 functions to activate TGF-βs, is atypical. Its β8 subunit binds to a distinctive cytoskeleton adaptor and does not exhibit large changes in conformation upon binding to ligand. Here, crystal structures, hydrogen-deuterium exchange dynamics, and affinity measurements on mutants are used to compare αVβ8 and αVβ6. Lack of a binding site for one of three βI domain divalent cations and a unique β6-α7 loop conformation in β8 facilitate movements of the α1 and α1' helices at the ligand binding pocket toward the high affinity state, without coupling to β6-α7 loop reshaping and α7-helix pistoning that drive large changes in βI domain-hybrid domain orientation seen in other integrins. Reciprocal swaps between β6 and β8 βI domains increase affinity of αVβ6 and decrease affinity of αVβ8 and define features that regulate affinity of the βI domain and its coupling to the hybrid domain.
Organizational Affiliation:
Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, MA, USA.