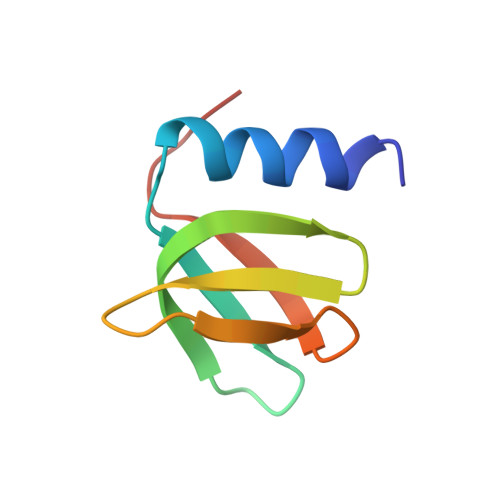
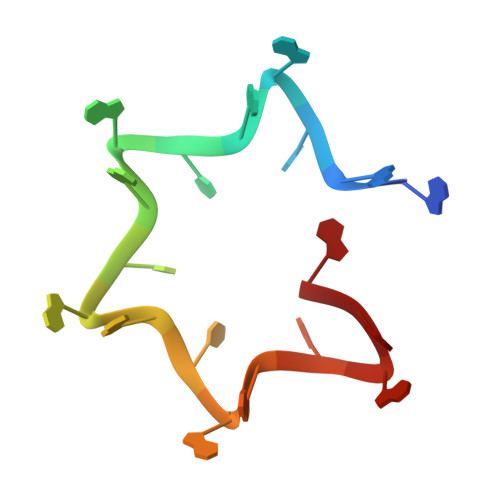
Architectural principles for Hfq/Crc-mediated regulation of gene expression
Pei, X.Y., Dendooven, T., Sonnleitner, E., Chen, S., Blasi, U., Luisi, B.F.(2019) Elife 8
- PubMed: 30758287
- DOI: https://doi.org/10.7554/eLife.43158
- Primary Citation of Related Structures:
6O1K, 6O1L, 6O1M - PubMed Abstract:
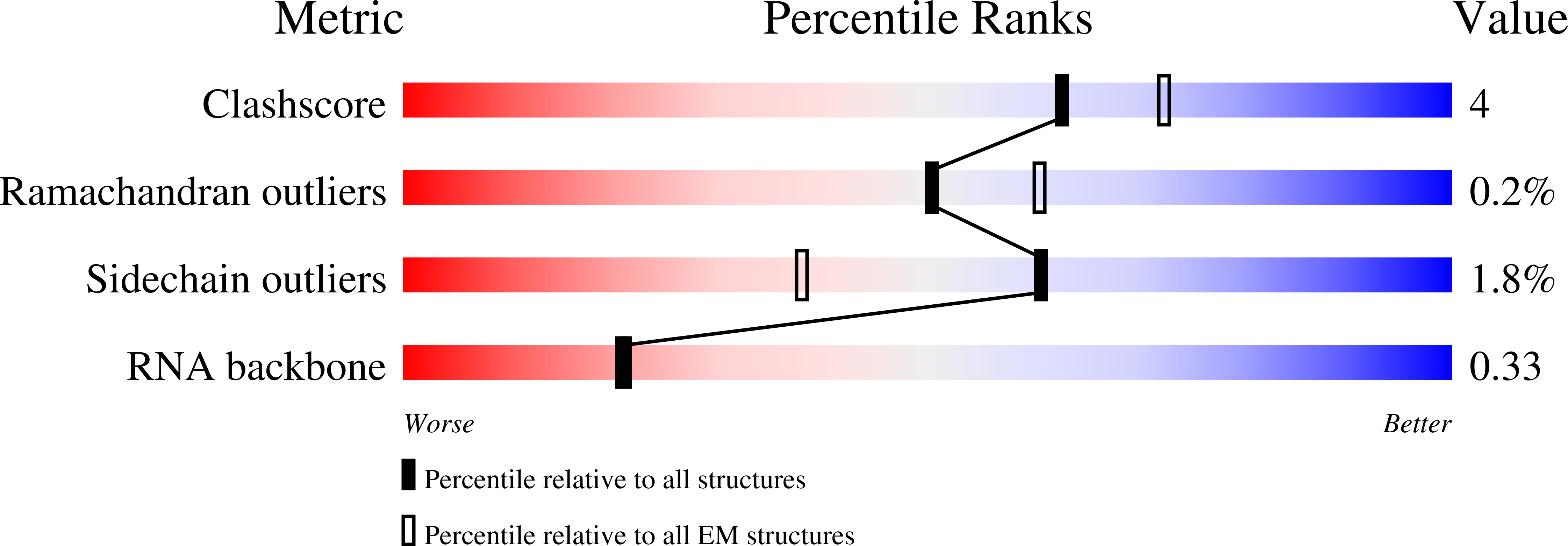
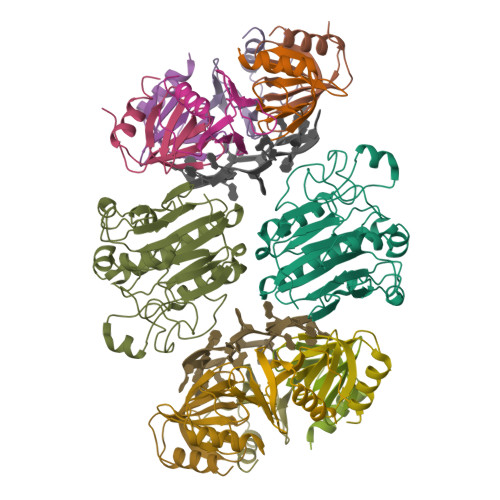
In diverse bacterial species, the global regulator Hfq contributes to post-transcriptional networks that control expression of numerous genes. Hfq of the opportunistic pathogen Pseudomonas aeruginosa inhibits translation of target transcripts by forming a regulatory complex with the catabolite repression protein Crc. This repressive complex acts as part of an intricate mechanism of preferred nutrient utilisation. We describe high-resolution cryo-EM structures of the assembly of Hfq and Crc bound to the translation initiation site of a target mRNA. The core of the assembly is formed through interactions of two cognate RNAs, two Hfq hexamers and a Crc pair. Additional Crc protomers are recruited to the core to generate higher-order assemblies with demonstrated regulatory activity in vivo. This study reveals how Hfq cooperates with a partner protein to regulate translation, and provides a structural basis for an RNA code that guides global regulators to interact cooperatively and regulate different RNA targets.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom.