Structural Study of Agmatine Iminohydrolase FromMedicago truncatula, the Second Enzyme of the Agmatine Route of Putrescine Biosynthesis in Plants.
Sekula, B., Dauter, Z.(2019) Front Plant Sci 10: 320-320
- PubMed: 30984210
- DOI: https://doi.org/10.3389/fpls.2019.00320
- Primary Citation of Related Structures:
6NIB, 6NIC - PubMed Abstract:
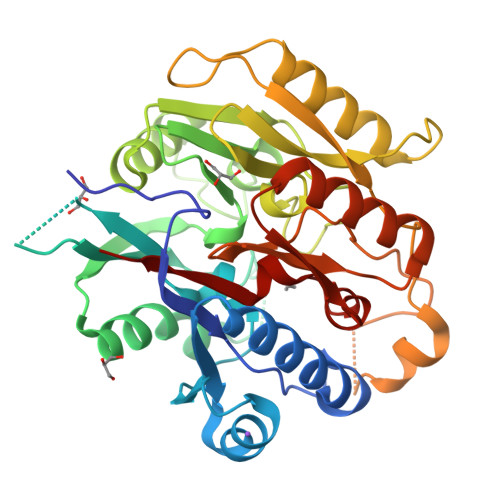
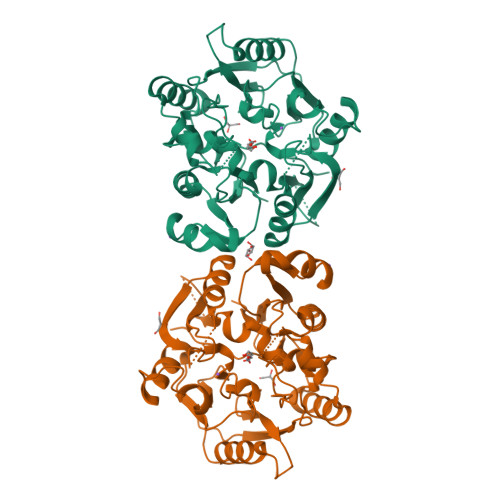
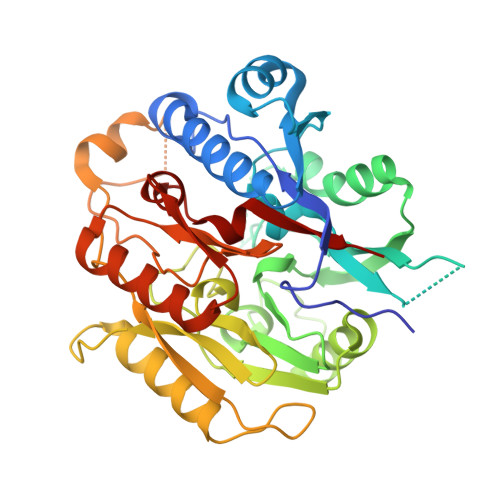
Plants are unique eukaryotes that can produce putrescine (PUT), a basic diamine, from arginine via a three-step pathway. This process starts with arginine decarboxylase that converts arginine to agmatine. Then, the consecutive action of two hydrolytic enzymes, agmatine iminohydrolase (AIH) and N- carbamoylputrescine amidohydrolase, ultimately produces PUT. An alternative route of PUT biosynthesis requires ornithine decarboxylase that catalyzes direct putrescine biosynthesis. However, some plant species lack this enzyme and rely only on agmatine pathway. The scope of this manuscript concerns the structural characterization of AIH from the model legume plant, Medicago truncatula . Mt AIH is a homodimer built of two subunits with a characteristic propeller fold, where five αββαβ repeated units are arranged around the fivefold pseudosymmetry axis. Dimeric assembly of this plant AIH, formed by interactions of conserved structural elements from one repeat, is drastically different from that observed in dimeric bacterial AIHs. Additionally, the structural snapshot of Mt AIH in complex with 6-aminohexanamide, the reaction product analog, presents the conformation of the enzyme during catalysis. Our structural results show that Mt AIH undergoes significant structural rearrangements of the long loop, which closes a tunnel-shaped active site over the course of the catalytic event. This conformational change is also observed in AIH from Arabidopsis thaliana , indicating the importance of the closed conformation of the gate-keeping loop for the catalysis of plant AIHs.
Organizational Affiliation:
Synchrotron Radiation Research Section of Macromolecular Crystallography Laboratory, National Cancer Institute, Argonne, IL, United States.