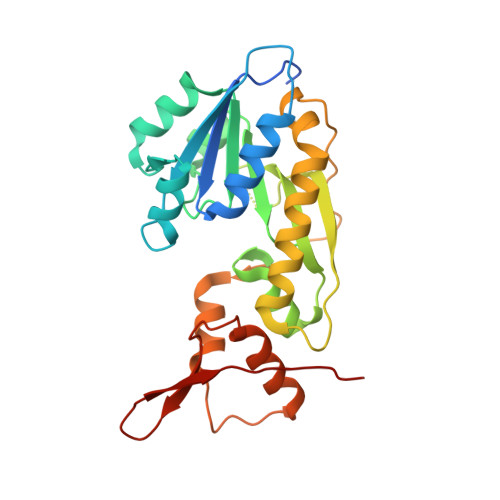
Investigation of a sugar N-formyltransferase from the plant pathogen Pantoea ananatis.
Hofmeister, D.L., Thoden, J.B., Holden, H.M.(2019) Protein Sci 28: 707-716
- PubMed: 30666752
- DOI: https://doi.org/10.1002/pro.3577
- Primary Citation of Related Structures:
6NBP - PubMed Abstract:
Pantoea ananatis is a Gram-negative bacterium first recognized in 1928 as the causative agent of pineapple rot in the Philippines. Since then various strains of the organism have been implicated in the devastation of agriculturally important crops. Some strains, however, have been shown to function as non-pathogenic plant growth promoting organisms. To date, the factors that determine pathogenicity or lack thereof between the various strains are not well understood. All P. ananatis strains contain lipopolysaccharides, which differ with respect to the identities of their associated sugars. Given our research interest on the presence of the unusual sugar, 4-formamido-4,6-dideoxy-d-glucose, found on the lipopolysaccharides of Campylobacter jejuni and Francisella tularensis, we were curious as to whether other bacteria have the appropriate biosynthetic machinery to produce these unique carbohydrates. Four enzymes are typically required for their biosynthesis: a thymidylyltransferase, a 4,6-dehydratase, an aminotransferase, and an N-formyltransferase. Here, we report that the gene SAMN03097714_1080 from the P. ananatis strain NFR11 does, indeed, encode for an N-formyltransferase, hereafter referred to as PA1080c. Our kinetic analysis demonstrates that PA1080c displays classical Michaelis-Menten kinetics with dTDP-4-amino-4,6-dideoxy-d-glucose as the substrate and N 10 -formyltetrahydrofolate as the carbon source. In addition, the X-ray structure of PA1080c, determined to 1.7 Å resolution, shows that the enzyme adopts the molecular architecture observed for other sugar N-formyltransferases. Analysis of the P. ananatis NFR11 genome suggests that the three other enzymes necessary for N-formylated sugar biosynthesis are also present. Intriguingly, those strains of P. ananatis that are non-pathogenic apparently do not contain these genes.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, Wisconsin, 53706.