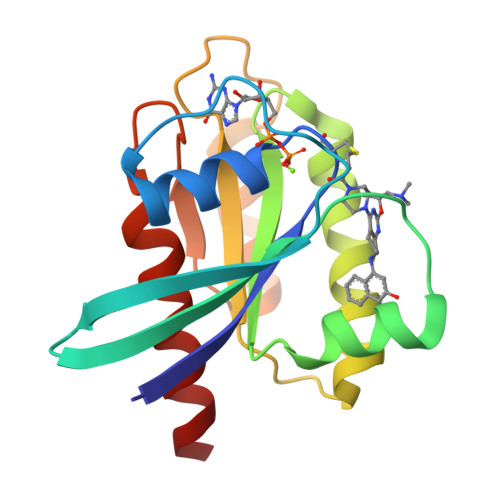
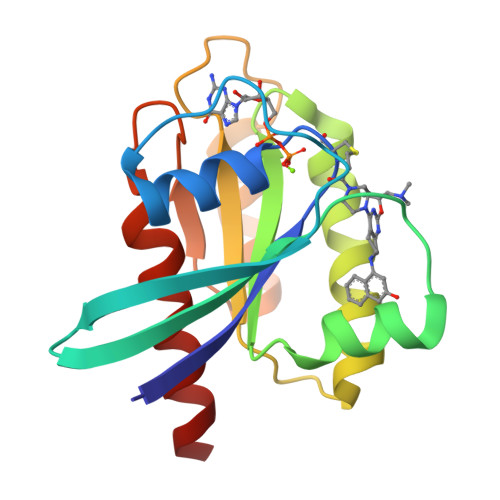
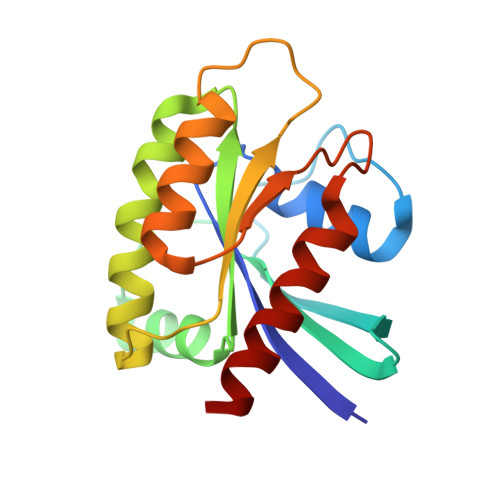
Discovery of Tetrahydropyridopyrimidines as Irreversible Covalent Inhibitors of KRAS-G12C with In Vivo Activity.
Fell, J.B., Fischer, J.P., Baer, B.R., Ballard, J., Blake, J.F., Bouhana, K., Brandhuber, B.J., Briere, D.M., Burgess, L.E., Burkard, M.R., Chiang, H., Chicarelli, M.J., Davidson, K., Gaudino, J.J., Hallin, J., Hanson, L., Hee, K., Hicken, E.J., Hinklin, R.J., Marx, M.A., Mejia, M.J., Olson, P., Savechenkov, P., Sudhakar, N., Tang, T.P., Vigers, G.P., Zecca, H., Christensen, J.G.(2018) ACS Med Chem Lett 9: 1230-1234
- PubMed: 30613331
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00382
- Primary Citation of Related Structures:
6N2J, 6N2K - PubMed Abstract:
KRAS is the most frequently mutated driver oncogene in human cancer, and KRAS mutations are commonly associated with poor prognosis and resistance to standard treatment. The ability to effectively target and block the function of mutated KRAS has remained elusive despite decades of research. Recent findings have demonstrated that directly targeting KRAS-G12C with electrophilic small molecules that covalently modify the mutated codon 12 cysteine is feasible. We have discovered a series of tetrahydropyridopyrimidines as irreversible covalent inhibitors of KRAS-G12C with in vivo activity. The PK/PD and efficacy of compound 13 will be highlighted.
Organizational Affiliation:
Array BioPharma, Inc., 3200 Walnut Street, Boulder, Colorado 80301, United States.