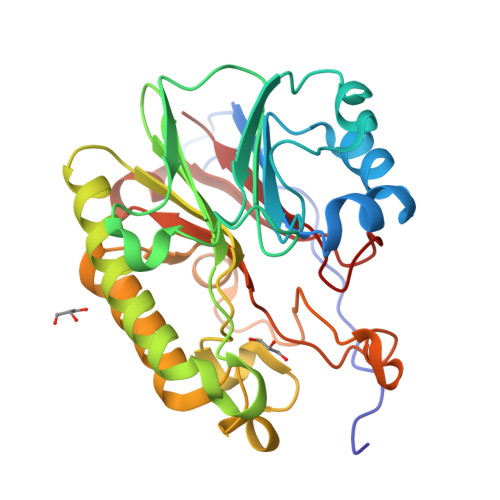
Discovery of Macrocyclic Inhibitors of Apurinic/Apyrimidinic Endonuclease 1.
Trilles, R., Beglov, D., Chen, Q., He, H., Wireman, R., Reed, A., Chennamadhavuni, S., Panek, J.S., Brown, L.E., Vajda, S., Porco Jr., J.A., Kelley, M.R., Georgiadis, M.M.(2019) J Med Chem 62: 1971-1988
- PubMed: 30653918
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01529
- Primary Citation of Related Structures:
6MK3, 6MKK, 6MKM, 6MKO - PubMed Abstract:
Apurinic/apyrimidinic endonuclease 1 (APE1) is an essential base excision repair enzyme that is upregulated in a number of cancers, contributes to resistance of tumors treated with DNA-alkylating or -oxidizing agents, and has recently been identified as an important therapeutic target. In this work, we identified hot spots for binding of small organic molecules experimentally in high resolution crystal structures of APE1 and computationally through the use of FTMAP analysis ( http://ftmap.bu.edu/ ). Guided by these hot spots, a library of drug-like macrocycles was docked and then screened for inhibition of APE1 endonuclease activity. In an iterative process, hot-spot-guided docking, characterization of inhibition of APE1 endonuclease, and cytotoxicity of cancer cells were used to design next generation macrocycles. To assess target selectivity in cells, selected macrocycles were analyzed for modulation of DNA damage. Taken together, our studies suggest that macrocycles represent a promising class of compounds for inhibition of APE1 in cancer cells.
Organizational Affiliation:
Department of Chemistry and Center for Molecular Discovery (BU-CMD) , Boston University , Boston , Massachusetts 02215 , United States.