Structure and Dynamics of Stacking Interactions in an Antibody Binding Site.
Adhikary, R., Zimmermann, J., Stanfield, R.L., Wilson, I.A., Yu, W., Oda, M., Romesberg, F.E.(2019) Biochemistry 58: 2987-2995
- PubMed: 31243995
- DOI: https://doi.org/10.1021/acs.biochem.9b00119
- Primary Citation of Related Structures:
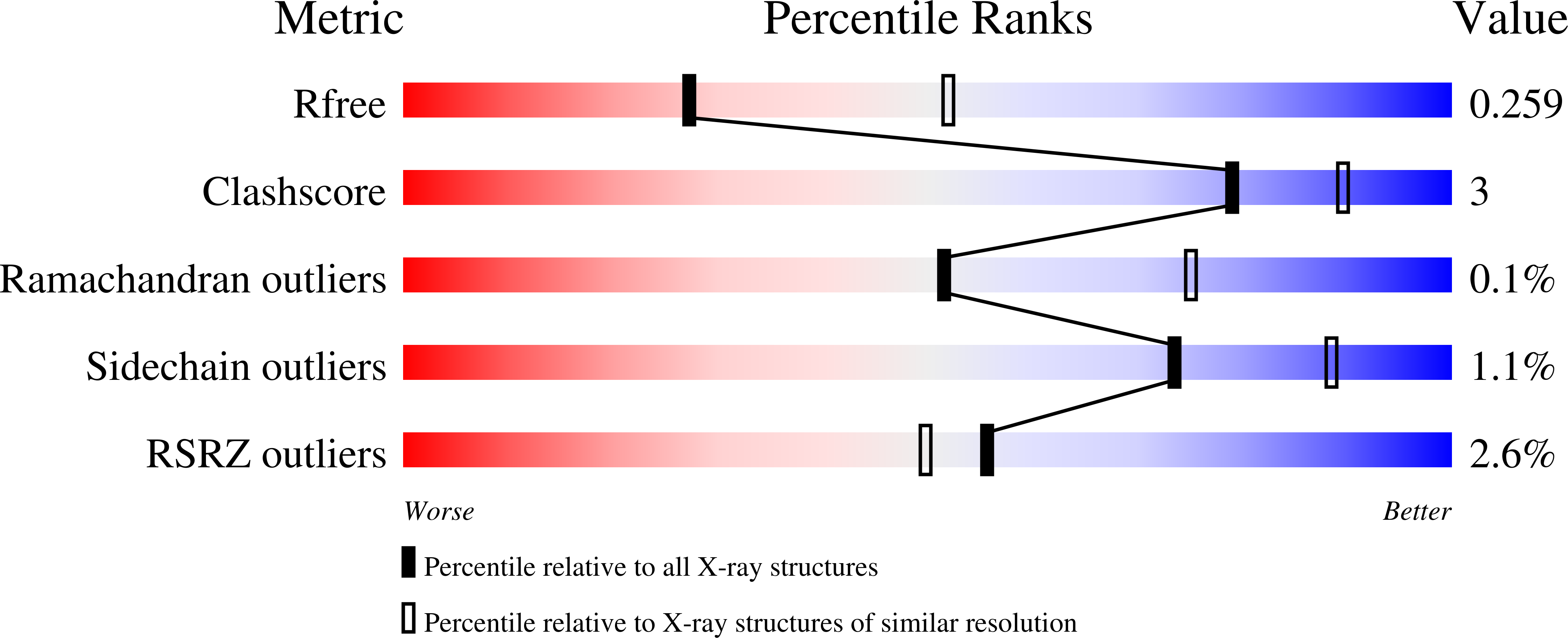
6M87 - PubMed Abstract:
For years, antibodies (Abs) have been used as a paradigm for understanding how protein structure contributes to molecular recognition. However, with the ability to evolve Abs that recognize specific chromophores, they also have great potential as models for how protein dynamics contribute to molecular recognition. We previously raised murine Abs to different chromophores and, with the use of three-pulse photon echo peak shift spectroscopy, demonstrated that the immune system is capable of producing Abs with widely varying flexibility. We now report the characterization of the complexes formed between two Abs, 5D11 and 10A6, and the chromophoric ligand that they were evolved to recognize, 8-methoxypyrene-1,3,6-trisulfonic acid (MPTS). The sequences of the Ab genes indicate that they evolved from a common precursor. We also used a variety of spectroscopic methods to probe the photophysics and dynamics of the Ab-MPTS complexes and found that they are similar to each other but distinct from previously characterized anti-MPTS Abs. Structural studies revealed that this difference likely results from a unique mode of binding in which MPTS is sandwiched between the side chain of Phe H 98, which interacts with the chromophore via T-stacking, and the side chain of Trp L 91, which interacts with the chromophore via parallel stacking. The T-stacking interaction appears to mediate relaxation on the picosecond time scale, while the parallel stacking appears to mediate relaxation on an ultrafast, femtosecond time scale, which dominates the response. The anti-MPTS Abs thus not only demonstrate the simultaneous use of the two limiting modes of stacking for molecular recognition, but also provide a unique opportunity to characterize how dynamics might contribute to molecular recognition. Both types of stacking are common in proteins and protein complexes where they may similarly contribute to dynamics and molecular recognition.
Organizational Affiliation:
Graduate School of Life and Environmental Sciences , Kyoto Prefectural University , 1-5, Hangi-cho , Shimogamo, Sakyo-ku, Kyoto 606-8522 , Japan.