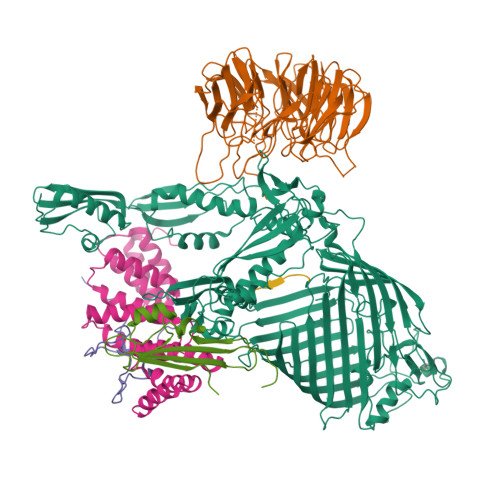
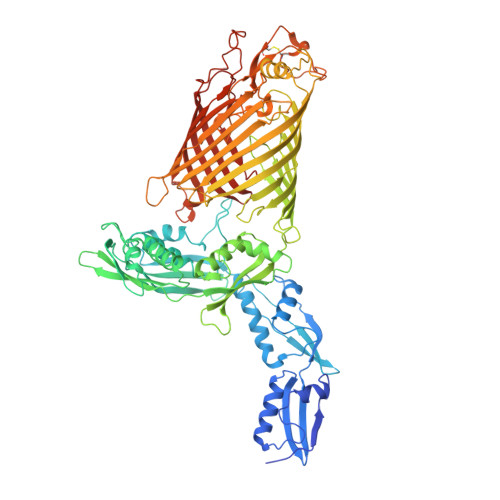
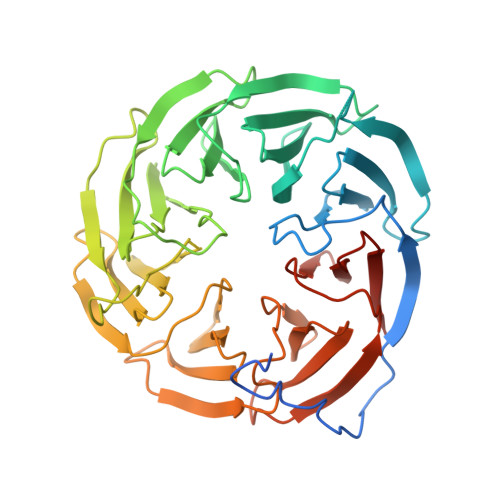
Structures of the beta-barrel assembly machine recognizing outer membrane protein substrates.
Xiao, L., Han, L., Li, B., Zhang, M., Zhou, H., Luo, Q., Zhang, X., Huang, Y.(2021) FASEB J 35: e21207-e21207
- PubMed: 33368572
- DOI: https://doi.org/10.1096/fj.202001443RR
- Primary Citation of Related Structures:
6LYQ, 6LYR, 6LYS, 6LYU - PubMed Abstract:
β-barrel outer membrane proteins (β-OMPs) play critical roles in nutrition acquisition, protein import/export, and other fundamental biological processes. The assembly of β-OMPs in Gram-negative bacteria is mediated by the β-barrel assembly machinery (BAM) complex, yet its precise mechanism remains elusive. Here, we report two structures of the BAM complex in detergents and in nanodisks, and two crystal structures of the BAM complex with bound substrates. Structural analysis indicates that the membrane compositions surrounding the BAM complex could modulate its overall conformations, indicating low energy barriers between different conformational states and a highly dynamic nature of the BAM complex. Importantly, structures of the BAM complex with bound substrates and the related functional analysis show that the first β-strand of the BamA β-barrel (β1 BamA ) in the BAM complex is associated with the last but not the first β-strand of a β-OMP substrate via antiparallel β-strand interactions. These observations are consistent with the β-signal hypothesis during β-OMP biogenesis, and suggest that the β1 BamA strand in the BAM complex may interact with the last β-strand of an incoming β-OMP substrate upon their release from the chaperone-bound state.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.