A novel selective autophagy receptor, CCDC50, delivers K63 polyubiquitination-activated RIG-I/MDA5 for degradation during viral infection.
Hou, P., Yang, K., Jia, P., Liu, L., Lin, Y., Li, Z., Li, J., Chen, S., Guo, S., Pan, J., Wu, J., Peng, H., Zeng, W., Li, C., Liu, Y., Guo, D.(2021) Cell Res 31: 62-79
- PubMed: 32612200
- DOI: https://doi.org/10.1038/s41422-020-0362-1
- Primary Citation of Related Structures:
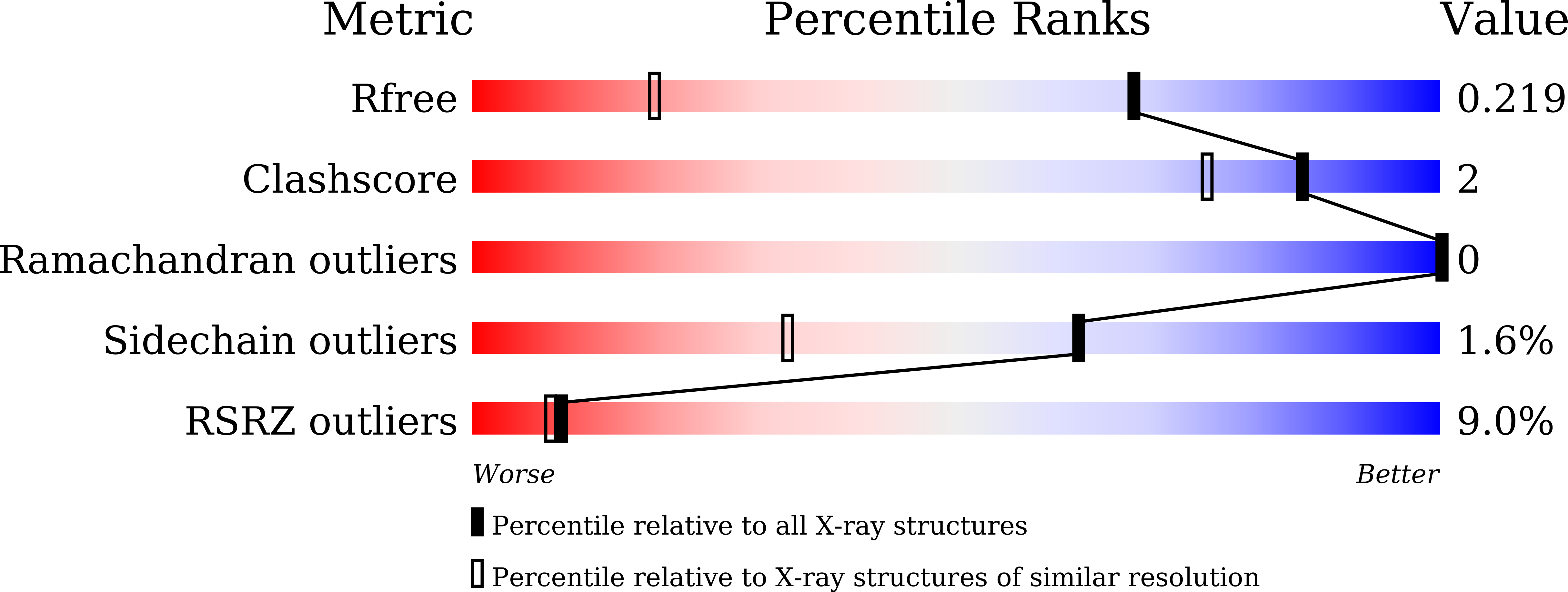
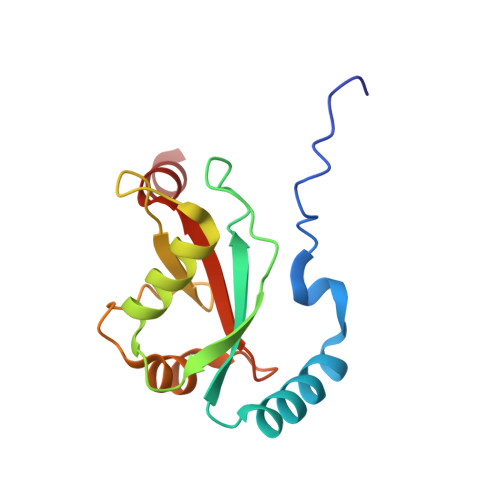
6LAN - PubMed Abstract:
Autophagy is a conserved process that delivers cytosolic substances to the lysosome for degradation, but its direct role in the regulation of antiviral innate immunity remains poorly understood. Here, through high-throughput screening, we discovered that CCDC50 functions as a previously unknown autophagy receptor that negatively regulates the type I interferon (IFN) signaling pathway initiated by RIG-I-like receptors (RLRs), the sensors for RNA viruses. The expression of CCDC50 is enhanced by viral infection, and CCDC50 specifically recognizes K63-polyubiquitinated RLRs, thus delivering the activated RIG-I/MDA5 for autophagic degradation. The association of CCDC50 with phagophore membrane protein LC3 is confirmed by crystal structure analysis. In contrast to other known autophagic cargo receptors that associate with either the LIR-docking site (LDS) or the UIM-docking site (UDS) of LC3, CCDC50 can bind to both LDS and UDS, representing a new type of cargo receptor. In mouse models with RNA virus infection, CCDC50 deficiency reduces the autophagic degradation of RIG-I/MDA5 and promotes type I IFN responses, resulting in enhanced viral resistance and improved survival rates. These results reveal a new link between autophagy and antiviral innate immune responses and provide additional insights into the regulatory mechanisms of RLR-mediated antiviral signaling.
Organizational Affiliation:
MOE Key Laboratory of Tropical Disease Control, Centre for Infection and Immunity Studies (CIIS), Seventh Affiliated Hospital, School of Medicine, Sun Yat-sen University, Shenzhen, Guangdong, 518107, China.