Structural and mechanistic insights into Quinolone Synthase to address its functional promiscuity
Mallika, V., Abhinav, K.V., Frandsen, K.E.H., Soniya, E.V.(2024) Commun Biol
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2024) Commun Biol
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Type III polyketide synthase | 391 | Aegle marmelos | Mutation(s): 0 | 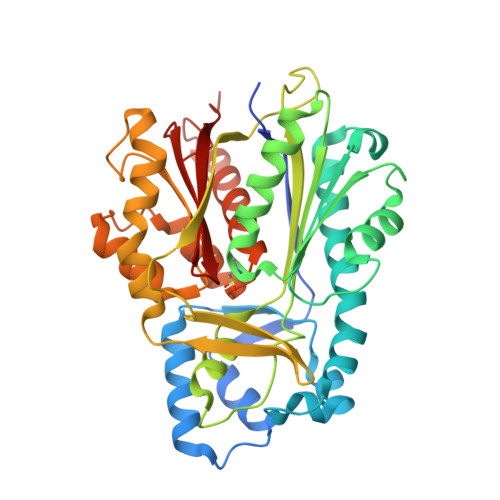 | |
UniProt | |||||
Find proteins for M1HE54 (Aegle marmelos) Explore M1HE54 Go to UniProtKB: M1HE54 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | M1HE54 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSD Query on CSD | A | L-PEPTIDE LINKING | C3 H7 N O4 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 149.84 | α = 90 |
| b = 149.84 | β = 90 |
| c = 105.491 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |
| PHASER | phasing |
| PDB_EXTRACT | data extraction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Council of Scientific & Industrial Research (CSIR) | India | 09/716(0178)/2018-EMR-1 dated 26.04.2018 |
| Novo Nordisk Foundation | Denmark | NNF21OC0071799 |