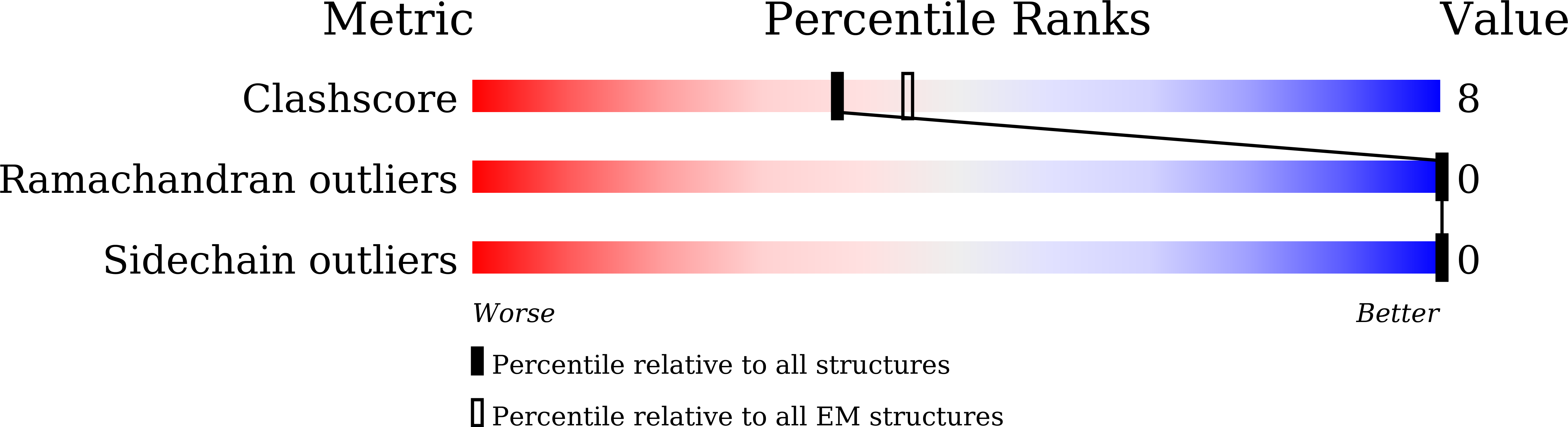Temperature-Resolved Cryo-EM Uncovers Structural Bases of Temperature-Dependent Enzyme Functions.
Chen, C.Y., Chang, Y.C., Lin, B.L., Huang, C.H., Tsai, M.D.(2019) J Am Chem Soc 141: 19983-19987
- PubMed: 31829582
- DOI: https://doi.org/10.1021/jacs.9b10687
- Primary Citation of Related Structures:
6KOU, 6KPA, 6KPE, 6KPH, 6KPI, 6KPJ, 6KPK, 6KQ4, 6KQ8, 6KQJ, 6KQK, 6KQO - PubMed Abstract:
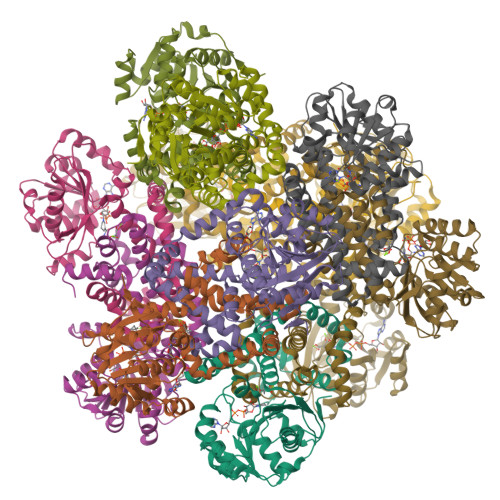
Protein functions are temperature-dependent, but protein structures are usually solved at a single (often low) temperature because of limitations on the conditions of crystal growth or protein vitrification. Here we demonstrate the feasibility of solving cryo-EM structures of proteins vitrified at high temperatures, solve 12 structures of an archaeal ketol-acid reductoisomerase (KARI) vitrified at 4-70 °C, and show that structures of both the Mg 2+ form (KARI:2Mg 2+ ) and its ternary complex (KARI:2Mg 2+ :NADH:inhibitor) are temperature-dependent in correlation with the temperature dependence of enzyme activity. Furthermore, structural analyses led to dissection of the induced-fit mechanism into ligand-induced and temperature-induced effects and to capture of temperature-resolved intermediates of the temperature-induced conformational change. The results also suggest that it is preferable to solve cryo-EM structures of protein complexes at functional temperatures. These studies should greatly expand the landscapes of protein structure-function relationships and enhance the mechanistic analysis of enzymatic functions.
Organizational Affiliation:
Department of Life Sciences , National Central University , Taoyuan 32001 , Taiwan.